Viral Hepatitis: Detection & Prevention
M3 India Newsdesk Dec 05, 2023
This comprehensive article delves into viral hepatitis, with a thorough understanding of each hepatitis strain, emphasising their distinct characteristics and shedding light on how they are diagnosed and prevented.
Case study
A 42-year-old patient with a medical history of no previous history of liver disease or chronic illnesses. Non-smoker and occasional alcohol consumer.
Case presentation
The patient reported experiencing fatigue, loss of appetite, jaundice (yellowing of the skin and eyes), and dark urine for the past two weeks. He visited his primary care physician due to the persistence of symptoms. Physical examination revealed jaundice and mild tenderness in the upper right abdomen. His blood tests indicated elevated liver enzymes (AST and ALT), suggesting liver inflammation.
Diagnostic investigations
- Blood tests- Mr. X underwent serological tests to detect specific viral markers. Results revealed high levels of hepatitis B surface antigen (HBsAg) and antibodies to hepatitis B core antigen (anti-HBc IgM), indicating an acute hepatitis B infection.
- Imaging studies- An abdominal ultrasound was conducted to assess the liver's condition, ruling out any structural abnormalities or complications such as cirrhosis or liver cancer.
- Liver biopsy (If required)- In some cases, a liver biopsy might be recommended to assess the extent of liver damage and guide treatment decisions. Upon confirming acute hepatitis B infection, the treatment approach focused on supportive care and managing symptoms.
Treatment Plan
- Rest and hydration: Advised adequate rest and increased fluid intake to support liver function.
- Avoidance of hepatotoxic substances: Instructed to refrain from alcohol consumption and certain medications that might further stress the liver.
- Monitoring: Regular follow-up visits were scheduled to monitor liver function tests and the progression of the infection.
- Antiviral therapy (if indicated): In certain cases, antiviral medications may be prescribed to suppress viral replication and reduce the risk of chronic infection.
- Patient outcome and follow-up: Over the subsequent weeks, Mr. X demonstrated gradual improvement in his symptoms. Follow-up blood tests indicated a decline in liver enzymes, suggesting resolution of the acute phase of hepatitis B infection. He was counselled on the importance of vaccination against hepatitis B to prevent future infections and transmission to others.
Viral hepatitis
Viral hepatitis is an Inflammation and damage of liver cells due to infection.
The liver is essential for removing toxins from the blood, storing vitamins, and producing hormones along with other functions. Viral hepatitis can disrupt these important processes, causing various health concerns.
Five main viruses cause viral hepatitis, termed A, B, C, D and E.
Clinical manifestations
Symptoms can include:
- Yellowing skin and eyes
- Dark urine
- Clay-coloured stools - 1-5 days before the onset of clinical jaundice
- Nausea and vomiting
- Loss of appetite
- Extreme fatigue
- Low Grade fever (38-39 °C) - HAV and HEV
Onset of jaundice
- Constitutional symptoms diminish
- The liver is enlarged and tender
- Right upper quadrant pain and discomfort
Recovery/ post-icteric phase
- Constitutional symptoms disappear
- 2-12 weeks (more prolonged in HBV and HCV)
- Some liver enlargement and biochemical test abnormalities are still evident
- Complete clinical and biochemical recovery- 1 - 2 months
Several clinical syndromes may develop after exposure to hepatitis viruses
- Acute hepatitis
- Fulminant hepatitis
- Chronic hepatitis
- Chronic carrier state
Pathology
The typical morphologic lesions of all types of viral hepatitis are similar:
- Panlobular infiltration with mononuclear cells
- Hepatic cell necrosis
- Hyperplasia of Kupffer cells
- Variable degree of cholestasis
Hepatitis A
- ss RNA
- Enterovirus 72
- Family : Picornavirus
- Genus: Hepatovirus
- Nonenveloped 27 nm, heat, acid and ether-resistant RNA virus
Inactivation by
- Boiling for 1 min
- Contact with formaldehyde
- Chlorine
- Ultraviolet radiation
Epidemiology
- Transmission- Fecal - oral route.
- Incubation period: 4 weeks/ 15-45 days.
- Replication - Limited in the liver.
- Late Incubation period: virus is present in the liver, bile, stools and blood.
- Period of communicability: before to after symptoms appear.
Diagnosis
Serology
- IgM anti-HAV: Acute HAV infection
- IgG anti-HAV: Resolution of the previous infection
Treatment and prevention
Prophylaxis with immune serum globulin given before or early in the incubation period (80% to 90% effective in preventing clinical illness)
Post-exposure: administration of 0.02 mL/kg.
Active immunisation is recommended for:
- Travellers to developing countries: < 3 months travel: 0.02 mL/kg.
- Frequent travellers: 0.06 mL/kg every 4-6 months.
- Children 2-18 years old.
Hep A vaccine
- Formalin - Inactivated Vaccines.
- Made from strains of HAV attenuated.
- Tissue cultures are safe, immunogenic and effective in preventing hepatitis A.
- Hepatitis A Vaccination provides long-lasting protection (Protective levels of HAV should last 20 years after Vaccination).
Hepatitis B
- Partially dsDNA
- Viral family – Hepadnavirus
- Incubation Period – 4 to 26 weeks
High-risk populations for whom HBV screening is recommended:
- People born in countries/regions with a high (≥8%) and intermediate (≥2%) prevalence of HBV infection including immigrants and adopted children and including persons born in the United States who were not vaccinated as infants and whose parents emigrated from areas of high HBV endemicity.
- Household and sexual contact of persons with hepatitis B.
- Babies born to HBsAg-positive mothers.
- Persons who have used injection drugs.
- Persons with multiple sexual contacts or a history of sexually transmitted disease.
- Men who have sex with men.
- Inmates of correctional facilities.
- Persons with elevated alanine and aspartate aminotransferase levels.
- Blood/Plasma/ Organ/ Tissue/ Semen donors.
- Persons with HCV or HIV infection.
- Hemodialysis patients.
- Pregnant women.
- Persons who are the source of blood or body fluids that would be an indication for Postexposure Prophylaxis (eg. needles stick, mucosal exposure, Sexual assault).
- Persons who require immunosuppressive or cytotoxic therapy.
Diagnosis and interpretation
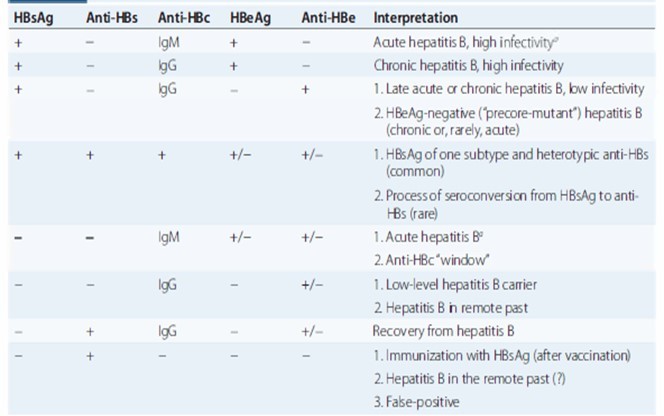
Prevention
Pre-exposure hepatitis B Vaccination schedules
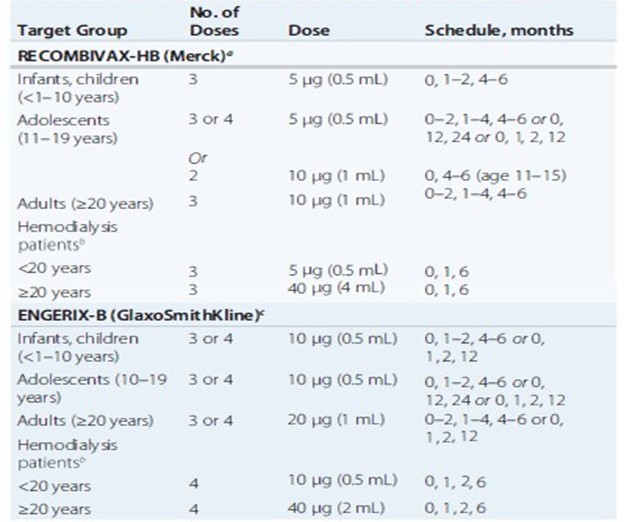
Hepatitis C
- Major cause of liver disease worldwide
- The most common chronic blood-borne infection
- Most common cause of Chronic liver disease
The most common risk factors for HCV are:
- Intravenous drug abuse
- Multiple sex partners
- Having had surgery within the past 6 months
- Needle stick injury
- Multiple contacts with and HCV infected person
- Employment in medical and dental fields
- Virology
HCV
- Single-stranded positive sense RNA virus.
- Belongs to the FLAVIVIRIDAE Family.
- The sole member of the genus Hepacivirus.
- The genome of HCV contains approximately 9600 nucleotides with an open reading frame (ORF) that encodes one large viral polypeptide. precursor of 3008 to 3033 amino acids.
Hepatocyte- Major site of viral replication
Small enveloped, single-stranded RNA virus with 9.6 kilobase that codes for a single polyprotein with one open reading frame, which is processed into functional proteins.
Genotypes and quasispecies
1. Viral genotype
- The first division is used to describe the genetic heterogeneity of HCV.
- Refers to genetically distinct groups of HCV isolates that have arisen during the evolution of the virus.
- The sequence clusters into 6 major genotypes.
2. Diagnosis
Presence of anti-HCV antibodies- Indicates exposure to virus
3. Virologic assays
HCV RNA quantitative analysis - For confirming infection, monitoring response to treatment and evaluating immunocompromised patients
4. Prophylaxis
- Disposable equipment and rigorous sterilisation of reusable medical and surgical equipment have reduced nosocomial HCV infection.
- Avoid having sexual intercourse with multiple partners.
Prevention of hepatitis B and C
- Exclusion of commercial blood donors and reliance on volunteer blood donors.
- Screening donor blood with surrogate markers such as HBsAg, anti-HCV, HIV.
Treatment
- Eradication of the virus - Primary goal of therapy for HCV infections.
- Sustained Virologic Response (SVR) - Absence of detectable virus in blood 24 hrs after the completion of therapy- an excellent marker for the resolution of HCV infection.
Hepatitis D
- Discovered in 1977
- The only member of Deltavirus
- HDV is a defective RNA virus that co-infects with and requires the helper function of HBV for its replication and expression
Forms
1. Large delta antigen - 214 amino acids.
2. Small delta antigen
- 195 amino acid species
- Plays a role in facilitating HDV RNA replication
- HDV is a satellite virus which binds to the capsid of the Hepatitis B virus
Superinfection
Superinfect a person already infected with HBV.
Co-infection
- HDV can infect a person simultaneously with HBV.
- It is clinically indistinguishable from other forms of viral hepatitis.
- 90% of patients are asymptomatic.
- The incubation period is 21-45 days but may be shorter in cases of superinfection.
Prevention
- Vaccinating susceptible persons with the hepatitis B vaccine.
- No products are available for immunoprophylaxis to prevent HbsAg carriers.
- Avoidance of percutaneous exposure and limitation of intimate contact with persons who have HDV infection.
Hepatitis E
- Virus Particle: 32 - 34 nm
- Morphology: Nonenveloped, icosahedral
- Genome: 7.6 kb RNA, linear, SS+
- Classification – Hepeviridae family
- Antigen - HEV antigen
- Antibodies - Anti HEV
Agent of enterically transmitted hepatitis.
Rare in USA, Occurs in Asia, Mediterranean countries, and Central America.
Diagnosis
- IgM/IgG anti-HEV (assays not routinely available)
- Virus in stool, bile, hepatocyte cytoplasm
- It has 3 open reading frames (ORF) genes; ORF 1, ORF 2, ORF 3
Epidemiology
- Resembles Hepatitis A virus (HAV) in its primarily enteric mode of spread
- Commonly on contaminated water
- Rare person-to-person spread
- Infection arises in those populations that are immune to HAV and mostly young adults
Hepatitis A and E
Hepatitis A and E are most commonly spread through food and water that has come into contact with the faeces of a person with the virus.
Some ways of preventing infection include:
- Wash your hands carefully after using the bathroom and before eating.
- Ensuring that food is fully cooked and appropriately stored.
- Drinking only bottled water when travelling.
- Avoiding or peeling fruits and vegetables that may have been washed or grown in unsanitised water.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Jimmy Patel is a practising gastroenterologist in Chennai.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries