Standards of Care in Diabetes: ADA 2024 Updates
M3 India Newsdesk Jan 08, 2024
This article summarises some important updates in the management of diabetes and its complications from the American diabetes association standard care in Diabetes 2024.
Diagnosis of diabetes and prediabetes
HbA1c at the top of the testing hierarchy to acknowledge real–world practice when diagnosing diabetes and pre-diabetes.

Criteria for diagnosis of diabetes

Criteria for diagnosis of prediabetes
- A structured framework for investigations of suspected type1 diabetes in newly diagnosed adults.
- Standardised islet autoantibody tests are recommended for the classification of diabetes in adults who have phenotypic risk factors that overlap with those for Type 1 diabetes (T1D) (e.g., younger age at diagnosis, unintentional weight loss, ketoacidosis, or short time to insulin treatment).
- Screening for pre-symptomatic T1D may be done by detection of autoantibodies to insulin, glutamic acid decarboxylase (GAD), islet antigen 2 (IA-2), or zinc transporter 8 (ZnT8).

Screening for pre-diabetes and type 2 diabetes
- Testing for pre-diabetes or Type 2 diabetes (T2D) in asymptomatic people should be considered in adults of any age with overweight or obesity who have one or more risk factors. For all other people, screening should begin at the age of 35 years.
- Consider screening people for prediabetes or diabetes if on certain medications, such as glucocorticoids, statins, thiazide diuretics, some HIV medications, and second-generation antipsychotic medications, as these agents are known to increase the risk of these conditions.
Monogenic diabetes syndrome
- As per recommendations regardless of current age, all people diagnosed with diabetes in the first 6 months of life should have immediate genetic testing for neonatal diabetes.
- Children and young adults who do not have typical characteristics of type 1 or T2D and who often have a family history of diabetes in successive generations (suggestive of an autosomal dominant pattern of inheritance) should have genetic testing for maturity-onset diabetes of the young (MODY).

Prevention or delay of diabetes
- Adults with overweight or obesity at high risk of T2D should be referred for an intensive lifestyle behaviour change program to achieve and maintain a weight reduction of at least 7% of initial body weight through a healthy reduced-calorie diet and ≥ 150 min/week of moderate-intensity physical activity.
- Metformin for the prevention of T2D should be considered in adults at high risk of T2D, as typified by the DPP, especially those aged 25–59 years with BMI ≥35 kg/m2, higher fasting plasma glucose (e.g., ≥ 110 mg/dL [≥ 6 mmol/L]), and higher A1C (e.g., ≥ 6.0% [≥ 42 mmol/mol]), and in individuals with prior gestational diabetes mellitus.
- Teplizumab infusion to delay the onset of symptomatic T1D(stage 3) should be considered in selected individuals aged ≥8 years with stage 2 type 1 diabetes. Management should be in a specialised setting with appropriately trained personnel.
Emphasis on bone health in individuals with diabetes
- Fracture risk should be assessed in older adults with diabetes as a part of routine care in diabetes clinical practice, according to risk factors and comorbidities.
- Monitor bone mineral density using dual-energy X-ray absorptiometry of high-risk older adults with diabetes (aged >65 years) and younger individuals with diabetes and multiple risk factors every 2–3 years.
- Calcium and Vitamin D should be advised for preventing fractures in high-risk patients with diabetes. Anti-resorptive medications and osteoanabolic agents should be considered for people with diabetes who have low bone mineral density with a T-score ≤ -2.0 or have experienced fragility fractures.
Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH)
- Adults with T2D or prediabetes, particularly those with obesity or cardiometabolic risk factors or established CVD, should be screened/risk stratified for clinically significant liver fibrosis (defined as moderate fibrosis to cirrhosis) using a calculated fibrosis-4 index (FIB-4).
- Adults with T2D or prediabetes with an indeterminate or high FIB-4 should have additional risk stratification by liver stiffness measurement with transient elastography or the blood biomarker-enhanced liver fibrosis (ELF).
- For adults with T2D, particularly with overweight or obesity, with NAFLD lifestyle modification should be advised before using a GLP-1 receptor agonist with demonstrated benefits in NASH as an adjunctive therapy to lifestyle interventions for weight loss.
- Pioglitazone or GLP-1 receptor agonists are the preferred agents to treat such patients. Insulin is preferred in patients with decompensated cirrhosis. Statin therapy is safe to use in such patients.
- Consider metabolic surgery in appropriate candidates as an option to treat NASH in adults with T2D and to improve cardiovascular outcomes.
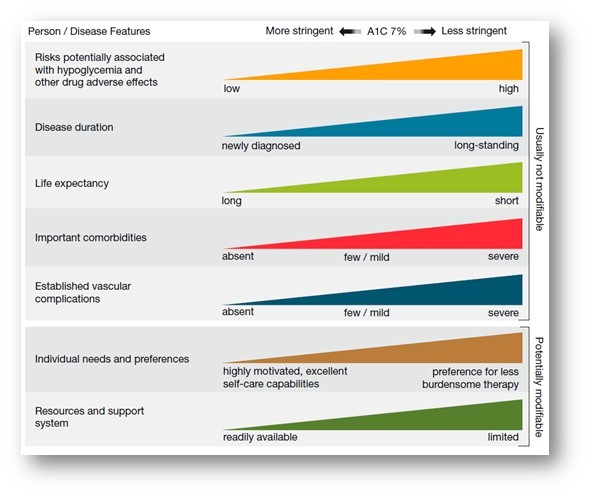
Re-evaluation of glycemic goals over time
- A finite period of intensive treatment to near-normal A1C may yield enduring benefits even if treatment is subsequently deintensified as characteristics change.
- Over time, comorbidities may emerge, decreasing life expectancy and thereby decreasing the potential to reap benefits from intensive treatment.
Cardiovascular disease and T2D
- If the patient is already on dual therapy or multiple glucose-lowering therapies and not on an SGLT2 inhibitor or a GLP-1 receptor agonist, consider switching to one of these agents with proven cardiovascular benefits.
- Introduce SGLT2 inhibitors or GLP-1 receptor agonists in people with CVD at A1C goal (independent of metformin) for cardiovascular benefit, independent of baseline A1C or individualised A1C goal.
Hypoglycemia assessment, prevention, and treatment
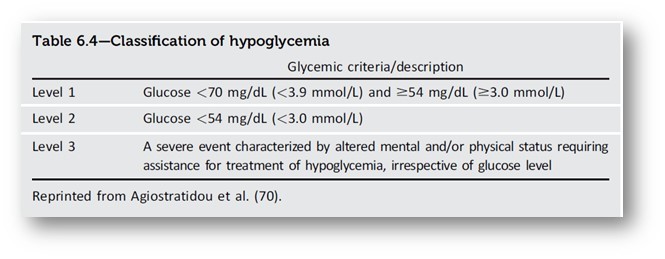
- The use of CGM is beneficial and recommended for individuals at high risk for hypoglycemia.
- Glucose is the preferred treatment for the conscious individual with glucose <70 mg/dL (<3.9 mmol/L), although any form of carbohydrate that contains glucose may be used. Fifteen minutes after initial treatment, repeat the treatment if hypoglycemia persists.
- Glucagon should be prescribed for all individuals taking insulin or at high risk for hypoglycemia.
Diabetes technology
- People with diabetes should be offered any type of diabetes device such as insulin pens, connected pens, glucose meters, and CGM or AID systems.
- As per recommendation treating physicians need to start CGM early in T1D to promote early achievement of glycemic goals.

Obesity and weight management for the prevention and treatment of diabetes
- Incorporation of additional anthropometric measurements beyond BMI (i.e., waist circumference, waist-to-hip ratio, and/or waist-to-height ratio) to encourage individualised assessments of body fat mass and distribution.
- Approaches to treating obesity should be individualised with intensive behavioural interventions, pharmacologic treatment, or metabolic surgery can be considered alone or in combination.
- People with diabetes and overweight or obese may benefit from any magnitude of weight loss. Weight loss of 3–7% of baseline weight improves glycemia and other intermediate cardiovascular risk factors.
Pharmacologic therapy for adults with T2D
- Early combination therapy can be considered in adults with T2D at treatment initiation to shorten the time to attainment of individualised treatment goals. Initial combination therapy should be considered in people presenting with A1C levels 1.5–2.0% above goal.
- Incorporation of high-glycemic-efficacy therapies or therapies for cardiovascular and kidney disease risk reduction(e.g., GLP-1RAs, dual GIP and GLP-1RA, and SGLT2 inhibitors) may allow for weaning of the current medication plan, particularly of agents that may increase the risk of hypoglycaemia and weight gain.
- For people with T2D and established ASCVD or indicators of high ASCVD risk, HF, or CKD, an SGLT2 inhibitor and/or GLP-1RA with demonstrated cardiovascular benefit is recommended as part of the glucose-lowering plan independent of A1C, independent of metformin use, and in consideration of person-specific factors.
- In adults with T2D, initiation of insulin should be considered regardless of background glucose-lowering therapy or disease stage if there is evidence of ongoing catabolism (e.g., unexpected weight loss), if symptoms of hyperglycemia are present, or when A1C or blood glucose levels are very high (i.e., A1C >10% [>86 mmol/mol] or blood glucose ≥300 mg/dL [≥ 16.7 mmol/L]).
- If insulin is used, combination therapy with a GLP-1 RA, including a dual GIP and GLP-1 RA, is recommended for greater glycemic effectiveness as well as beneficial effects on weight and hypoglycemia risk for adults with T2D. Avoid using glucose-lowering agents with higher hypoglycemia risk (i.e., sulfonylureas and meglitinides) along with insulin in elder patients.

CVD and risk management in patients with diabetes
As per recommendations, the reduction in diabetes complications can be achieved by lifestyle modification along with glycemia management, blood pressure management, lipid management and agents with CVD and kidney benefits.
Type 1 patients should be screened for complications like neuropathy and retinopathy within 5 years after the onset of diabetes and for type 2 patients at the time of diagnosis and at least annually thereafter.

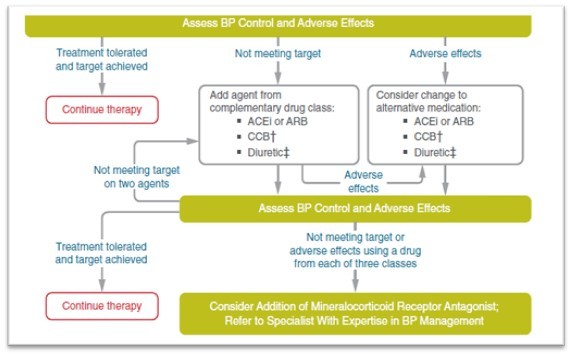
- Individuals with hypertension who are not meeting blood pressure targets on three classes of antihypertensive medications (including a diuretic) should be considered for MRA therapy.
- As per recommendation Statin plus fibrate or niacin combination therapy should be avoided, as it has no additional ASCVD benefits.
- Use aspirin therapy (75–162 mg/day) as a secondary prevention strategy in those with diabetes and a history of ASCVD. Clopidogrel is to be considered in patients allergic to aspirin.
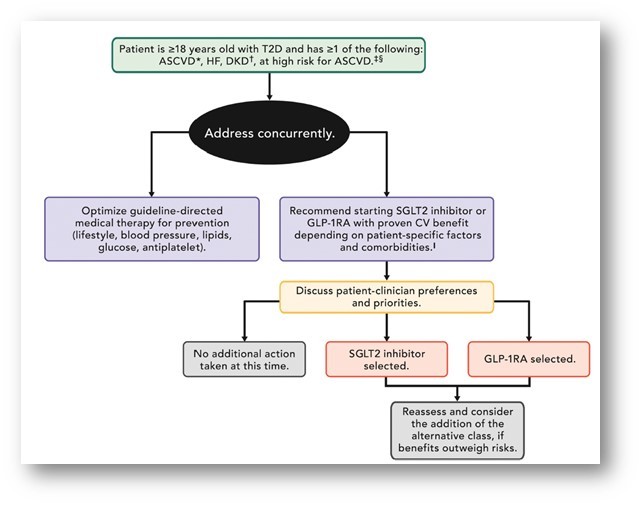
CKD and risk management in diabetes
- At least annually, urinary albumin (e.g., spot urinary albumin-to-creatinine ratio [UACR]) and eGFR should be assessed in people with T1D with a duration of ≥5 years and in all people with T2D regardless of treatment.
- Optimise glucose management and blood pressure control to reduce the risk or slow the progression of CKD and reduce CVD.
- In non-pregnant people with diabetes and hypertension, either an ACE inhibitor or ARB is recommended for those with moderately increased albuminuria (UACR 30–299 mg/g creatinine) and is strongly recommended for those with severely increased albuminuria (UACR ≥300 mg/g creatinine) and/or eGFR <60 mL/min/1.73 m2 to prevent the progression of CKD and reduce CVD.
- For people with T2D and CKD, the use of an SGLT2 inhibitor is recommended to reduce CKD progression and cardiovascular events in individuals with eGFR ≥ 20 mL/min/ 1.73 m2 and urinary albumin ≥ 200 mg/g creatinine.
- For CVD risk reduction in people with T2D and CKD, consider the use of an SGLT2 inhibitor, a GLP 1 agonist, or a non-steroidal mineralocorticoid receptor antagonist (MRA) (if eGFR is ≥ 25 mL/min/1.73 m2), monitor potassium with use of MRA.
- As per recommendation, the dietary protein intake should be aimed at a target level of 0.8 g/kg/day in non-dialysis CKD patients and 1.0–1.2 g/kg/day for patients who are on dialysis.
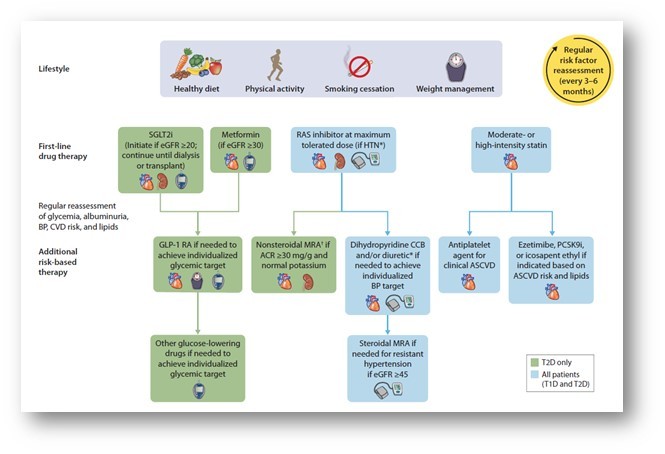
Management of diabetes patients with CKD
Retinopathy, neuropathy and foot care
As per recommendation, individuals with preexisting T1D or T2D should receive an eye exam before pregnancy and in the first trimester and should be monitored every trimester and for 1 year postpartum as indicated by the degree of retinopathy.
Panretinal laser photocoagulation therapy is indicated to reduce the risk of vision loss in individuals with high-risk PDR and, in some cases, severe non-proliferative diabetic retinopathy.
Intravitreous injections of anti-VEGF are indicated as first-line treatment for most eyes with diabetic macular oedema that involves the foveal centre and impairs vision acuity. In case of persistent macular edema after Anti VGEF injection consider macular focal/grid photocoagulation and intravitreal injections of corticosteroid.
As per recommendation gabapentinoids, SNRI, tricyclic antidepressants, and sodium channel blockers are prescribed as initial pharmacologic treatments for neuropathic pain in diabetes.
For foot care annual examination should include inspection of the skin, assessment of foot deformities, neurological assessment (10-g monofilament testing with at least one other assessment: pinprick, temperature, or vibration), and vascular assessment, including pulses in the legs and feet.
As per recommendation, initial screening for peripheral arterial disease (PAD) should include assessment of lower-extremity pulses, capillary refill time, rubor on dependency, pallor on elevation, and venous filling time.
Management of diabetes in pregnancy
- All pregnant individuals with diabetes should monitor fasting, pre-prandial, and postprandial blood glucose levels.
- Metformin and glyburide, individually or in combination, should not be used as first-line agents for treating hyperglycemia in pregnancy. Insulin is the preferred treatment for hyperglycemia in pregnancy. Metformin used for PCOS should be discontinued by the end of the first trimester.
- Individuals with GDM may also be candidates for aspirin therapy if they have a single high-risk factor or multiple moderate risk factors to lower the risk of preeclampsia.
Target for GDM
- Fasting glucose<95mg/dL (<5.3mmol/L) and either
- One-hour postprandial glucose <140 mg/dL (<7.8 mmol/L) or
- Two-hour postprandial glucose <120 mg/dL (<6.7 mmol/L)
Glycemic goals in hospitalised adults
Insulin and/or other therapies should be initiated or intensified for treatment of persistent hyperglycemia starting at a threshold of ≥180 mg/dL (≥ 10.0 mmol/L) (confirmed on two occasions within 24 h) for non-critically ill (non-ICU) individuals.
Once therapy is initiated, a glycemic goal of 140–180 mg/dL (7.8–10.0 mmol/L) is recommended for most critically ill (ICU) individuals with hyperglycemia.
To reduce surgical risk in people with diabetes, some institutions have A1C cutoffs for elective surgeries, and some have developed optimisation programs to lower A1C before surgery.
The following approach may be considered:
- The blood glucose goal in the perioperative period should be 100–180 mg/dL (5.6–10.0 mmol/L) within 4 h of the surgery. CGM should not be used alone for glucose monitoring during surgery.
- Metformin should be held on the day of surgery.
- SGLT2 inhibitors should be discontinued 3–4 days before surgery.
- Hold other oral glucose-lowering agents the morning of surgery or procedure and give one-half of the NPH dose or 75–80% doses of long-acting analogue insulin or adjust insulin pump basal rates based on the type of diabetes and clinical judgment.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Hitesh Saraogi is a diabetologist, physician and an obesity specialist at Dhanvantari Hospital, Raj Nagar Extension, Ghaziabad.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries