MoHFW-DGHS revises COVID-19 treatment guidelines, reduces drug use
M3 India Newsdesk Jun 14, 2021
COVID-19 has been in the talks for quite some time now. While there are many types of research happening around the globe, the DGHS has announced revisions to the guidelines for treating COVID-19. This article highlights the usage recommendations for and against some antibiotics and steroids and also helps with the treatment protocol in regards to relevant symptoms.
For our comprehensive coverage and latest updates on COVID-19 click here.
Introduction
As India's cities resume normalcy following a COVID peak, the Centre's Directorate General of Health Services (DGHS) has announced new COVID-19 treatment guidelines, defining the requirements for the use of certain medications in therapy. The revised guidelines significantly reduce the use of various antibiotics and steroids while cautioning against the overuse of remdesivir, tocilizumab, and other agents.
Treatment guidelines
- According to the DGHS guidelines, asymptomatic individuals should get no treatment, whereas mild cases should get antipyretic and antitussive medications in addition to inhalational budesonide.
- For mild cases, DGHS has discontinued the use of popular medications such as favipiravir, ivermectin, azithromycin, doxycycline, zinc, and plasma treatment.
- Similarly, DGHS recommends the use of steroids in moderate cases where SpO2 values fall below 92 per cent, as well as the use of anticoagulants and steroids.
- DGHS recommends rapid oxygen treatment, intubation and ventilation, as well as the use of steroids, anticoagulants, and immune modulators in severe instances.
- The DGHS has specified the use of remdesivir, indicating that it must be administered in selected moderate/severe COVID-19 patients hospitalised on supplemental oxygen within 10 days of illness, emphasising that it is an investigational medicine. Furthermore, the DGHS has cautioned against the use of tocilizumab in any except the most severe and severely sick patients.
- The nine-page guidelines also recommend the use of steroids in moderately severe and critically ill COVID-19 cases who are hospitalised.
- They also recommend performing a high-resolution CT (HRCT) scan of the chest only if suspected and confirmed cases of moderate COVID-19 continue to deteriorate clinically.
Numerous physicians have lauded the DGHS's straightforward and precise directions for reducing excessive medication use. Several physicians have emphasised the importance of DGHS-recommended measures such as the avoidance of antibiotics, anti-parasitic, plasma treatment, coronil, 2DG, favipiravir, and routine CT scans. Dr Sowmya Swaminathan, WHO's chief scientist, lauded the recommendations as well, urging the centre to issue them in all languages.
Comprehensive guidelines for management of COVID-19 patients
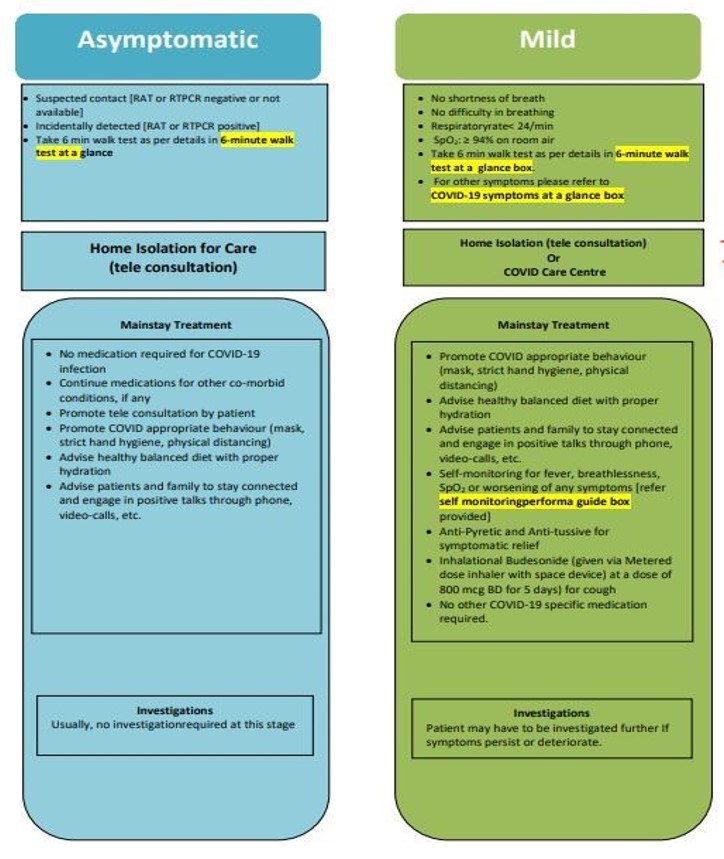

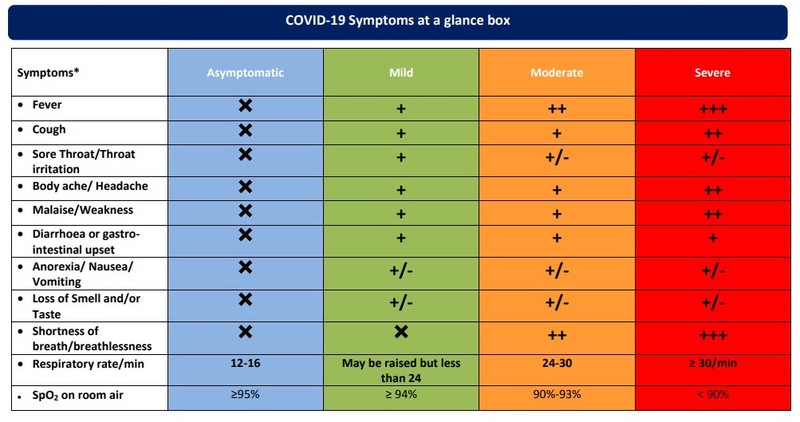
The monitoring sheet for COVID-19 patients at home has also been recommended to track the symptoms and manage the complications accordingly in time.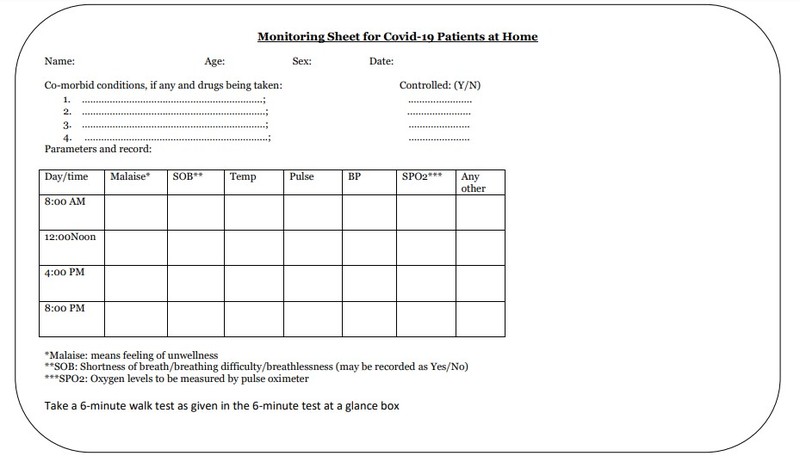
The 6-minute walk test
The 6-minute walk test has been emphasised to unmask hypoxia and detect the patients before deterioration.
How should a 6-minute walk test be conducted?
The 6-minute walk test is a well-established, straightforward clinical procedure for determining cardiopulmonary exercise tolerance. This test is used to reveal the presence of hypoxia. A patient with a pulse oximeter connected to his finger is instructed to walk for six minutes inside the boundaries of his room perpetually.
Any decrease in saturation below 94%, or an absolute decrease of more than 3% to 5%, or feeling sick (lightheaded, or significant findings like feeling out of breath) while doing the test or at the conclusion of the six minutes. These are the patients positive for the 6-minute walk test. Patients who have a positive 6-minute walk test may develop hypoxia, necessitating hospitalisation. It is advised to use for observation and oxygen supplementation. The test can be repeated every six to eight hours during home monitoring. It should not be performed on patients over the age of 70, on individuals with uncontrolled asthma, or on pregnant women.
Remdesivir usage recommendations
- Remdesivir is a restricted medicine licensed by DCG (I) under Emergency Use Authorisation solely on the basis of limited scientific evidence worldwide. It should only be used in COVID-19 patients who are hospitalised and those on supplemental oxygen within 10 days after commencement of the illness.
- It is not recommended for mild COVID-19 patients receiving home care or treatment in COVID care centres.
- Physicians are recommended to use remdesivir with extreme caution since it is still an investigational medicine with the potential to cause damage.
To prevent remdesivir usage, the following extra precautions are recommended:
- It must be recommended by senior faculty/specialists who are actively involved in the patient's treatment.
- If it needs to be advised/ordered during odd hours, the duty doctor should do so after consulting with a senior faculty member/specialist/unit in charge over the phone.
- Remdesivir advice/orders must be written and include the name, signature, and stamp of the treating physician.
- Every hospital should establish a Special Drug Committee (SDC) to assess remdesivir use in their facility on a regular basis. Wherever possible, it would be preferred to have a pharmacology professor/faculty member on the SDC.
- To promote sensible and appropriate use of remdesivir, the Special Drug Committee should discuss its results with practitioners on a regular basis.
- It should only be acquired and given by hospitals. Attendants/relatives of patients should not be requested to purchase remdesivir from the retail market.
Tocilizumab use recommendations
Tocilizumab is an immunosuppressive medication that has been approved by DCG (I) for use off-label in severe and critically sick COVID-19 patients who match the following criteria:
- If the patient's oxygen demand does not improve considerably after 24 to 48 hours of steroid therapy, and if the patient has considerably elevated inflammatory markers (C-reactive protein >75 mg/L), however, it is necessary to verify that the patient is clear of any bacterial, fungal, or tuberculous infection at the time of tocilizumab delivery.
- Dosage: 8 mg/kg body weight (maximum 800 mg) in 100 mL normal saline given over one hour.
Steroids use recommendations
- Steroids are not recommended and may be hazardous in asymptomatic or mild COVID-19 infection.
- Steroids are appropriate only in hospitalised COVID-19 patients who are moderately severe or severely unwell.
- Steroids should be taken at the appropriate time, at the appropriate dose, and for the appropriate duration.
- Self-medication with steroids is not recommended.
- Recommended dose: dexamethasone 6 mg IV once daily or orally once daily for the first 10 days or until discharge, whichever occurs first, based on clinical judgment on a daily basis.
- If dexamethasone is unavailable, an equivalent glucocorticoid dosage may be substituted with methylprednisolone 32 mg orally or 40 mg I/V or 50 mg hydrocortisone intravenously every 8 hours, or prednisone 40 mg (per oral).
- Blood glucose monitoring is required in all patients using steroids, since they may promote hyperglycaemia. In any event, infection with COVID-19 and its treatment is likely to cause diabetes in previously healthy individuals or exacerbate diabetes in previously diagnosed patients.
- It's also worth noting that steroids may prolong virus shedding, necessitating vigilance.
Anticoagulant use recommendations
Anticoagulant dosages in moderate cases: Prophylactic dosages of unfractionated heparin or low molecular weight heparin have to be administered in moderate cases of COVID-19 (weight-based, e.g., enoxaparin 0.5 mg/kg per day SC OD). There should be no contraindications to the procedure or a significant risk of bleeding.
In severe cases, prophylactic doses of unfractionated heparin or low molecular weight heparin (weight-based, e.g., enoxaparin 0.5 mg/kg per day SC OD) should be used in severe instances of COVID-19. Therapeutic dosages should be administered only if there is evidence of thrombosis. There should be no contraindications to the procedure or a significant risk of bleeding.
When to recommend HRCT
In individuals with COVID-19, a high-resolution CT (HRCT) scan of the chest offers a clearer picture of the degree and form of lung involvement. However, any new information gleaned from a chest HRCT scan is frequently insignificant in guiding treatment options. Treatment decisions are now made nearly completely on the basis of clinical severity and physiological impairment. As a result, treating doctors should use extreme caution when ordering HRCT imaging of the chest in COVID-19 patients.
Why is regular HRCT imaging of the chest not advised in COVID-19 patients?
- Nearly two-thirds of individuals who have asymptomatic COVID-19 exhibit nonspecific HRCT chest imaging abnormalities. The majority of them fail to advance clinically.
- In the initial week of sickness, HRCT imaging of the chest may frequently underestimate the level of lung involvement, creating a false sense of security.
- There is an imprecise correlation between the level of lung involvement as determined by HRCT imaging of the chest and hypoxia. Frequently, young people with significant lung involvement do not get hypoxia, whereas older people with minimal/less extensive lung involvement are more prone to suffer hypoxia.
- Repeated HRCT imaging may be related to an increased risk of developing cancer later in life. Situations in which HRCT imaging of the chest should not be performed include the following:
- HRCT chest scans should not be used to diagnose or test for COVID-19 infection. COVID-19 should be diagnosed exclusively by the use of authorised laboratory testing, as suggested by the ICMR.
- It is not advised in instances of COVID-19 that are asymptomatic or moderate.
- Treatment is not indicated in COVID-19 patients who have hypoxia and an abnormal chest radiograph.
- It is not necessary to measure therapy response. Frequently, despite the clinical recovery, lung lesions worsen radiographically.
Appropriate reasons for chest HRCT imaging in COVID-19 patients
Suspected and confirmed patients with moderate COVID-19 who continue to progress clinically despite the commencement of proper medication, particularly where the invasive fungal infection is a significant risk. Depending on the clinical assessment of the patient, the treating physician/intensive care physician may recommend HRCT chest. In light of the foregoing, treating doctors should take care when recommending chest HRCT imaging.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author is a practising super specialist from New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries