Malaria in monsoon: Recent updates
M3 India Newsdesk Jul 12, 2022
The diagnosis, clinical manifestations and treatment guidelines of malaria are penned down in this article.
Malaria
Malaria transmission may be controlled by providing prompt and efficient treatment. Chloroquine used to be an effective treatment for almost all types of malaria. Chloroquine-resistant P. falciparum malaria has been reported more often in recent research throughout the nation. The National Framework for Malaria Elimination in India 2016–2030 was created to address local concerns. Recently WHO has also recommended a malaria vaccine.
Clinical manifestations
Malaria is characterised by a high fever. It might be intermittent, periodic, or continuous. Chills and rigours are common symptoms. Headache, myalgia, arthralgia, anorexia, nausea, and vomiting are typical complaints associated with fever. Malaria symptoms can be non-specific and can be mistaken for other illnesses such as viral infections, enteric fever, and so on. Those who live in or recently visited an endemic region should be tested for malaria if they exhibit the aforementioned symptoms.
In the presence of symptoms such as a runny nose, cough, and other indicators of respiratory infection, diarrhoea/dysentery, burning micturition and/or lower abdomen discomfort, skin rash/infections, abscess, painful swelling of joints, lymphadenopathy, etc., alternative causes of fever should be addressed. All suspected cases of malaria should be examined by microscopy or a Rapid Diagnostic Test as soon as possible.
How malaria is diagnosed?
Microscopy
To confirm the diagnosis of malaria, microscopy of stained thick and thin blood smears is still preferred. Microscopy has a high level of sensitivity. At modest concentrations, malaria parasites may be detected. It may also be used to calculate the parasite burden. Different malaria parasite species and stages can be identified.
Rapid diagnostic test
Detection of parasite antigens in the blood is the basis for Rapid Diagnostic Tests (RDT). Many RDTs are available and each one has its own advantages. Some can exclusively identify P. falciparum, whereas others can also detect additional parasite species.
NVBDCP released bivalent RDTs for use in the public health sector, which can detect both P. falciparum and P. vivax. There may be discrepancies in the content and methodology of RDTs since they are made by various companies. Reading and following all directions in the user handbook is essential. Whenever the findings are ready, they should be viewed. Healthcare providers who are doing a fast malaria test must confirm that the kit they are using is up-to-current with its expiration date and has been carried out and kept according to the manufacturer's instructions.
Incorrect outcomes may be the consequence of not adhering to these standards. A good result from the Pf HRP-2-based tests may appear up to three weeks after a successful therapy and parasite removal. In these circumstances, the microscopic diagnosis should be linked with the findings.
Timely identification and therapy of malaria attempt to achieve the following goals:
- Full cure
- Prevention of simple malaria progressing to severe illness
- Prevention of mortality
- Interruption of transmission
- Minimisation of the possibility of drug-resistant parasites being selected and transmitted
The management of uncomplicated malaria
Treatment of P. vivax malaria
P. vivax patients should be treated with full therapeutic doses of 25 mg/kg of chloroquine, according to the age-based dosing regimen .P. vivax can trigger a relapse in some patients (8 to 30 per cent of the time). Primaquine, at a dose of 0.25 mg/kg body weight daily for 14 days, should be administered under medical supervision as a preventative measure.
Women who are pregnant, breastfeeding, or have a known G6PD deficiency should not use Primaquine. In G6PD deficiency, Primaquine can cause hemolysis. In places with a high frequency of G6PD deficiency, extreme caution should be maintained before providing any primaquine. It is important for the patient to promptly discontinue taking primaquine if he/she has any of the following symptoms :
- Dark coloured urine
- Yellow conjunctiva
- Blue discolouration of lips
- Stomach discomfort
- Nausea, vomiting
- Dyspnea
| Tablets | |||
| Age in years | Day 1 (10mg/kg) | Day 2 (10mg/kg) | Day 3 (5mg/kg) |
| < 1 | 1/2 | 1/2 | 1/4 |
| 1-4 | 1 | 1 | 1/2 |
| 5-8 | 2 | 2 | 1 |
| 9-14 | 3 | 3 | 1.5 |
| >15 | 4 | 4 | 2 |
Chloroquine for P. vivax
| Age in years | Daily dose in mg |
| <1 | Nil |
| 1-4 | 2.5 |
| 5-8 | 5 |
| 9-14 | 10 |
| >15 | 15 |
Primaquine for P. Vivax (Daily dose for 14 days)
Caution: Primaquine should be taken for 14 days under medical care, with the patient being educated on how to recognise danger signs. Pregnant women, newborns, and G6PD deficient persons should not be given Primaquine.
When administering primaquine, individual doctors must assess the potential hazards and advantages of the drug in light of variable recurrence rates, G6PD insufficiency, and testing facilities.
Management of P. falciparum malaria
All confirmed P. falciparum patients proven to be positive by microscopy or RDT should be treated with Artemisinin Combination Therapy (ACT). On Day 2, a single dosage of primaquine (0.75 mg/kg body weight) is to be administered. A long-acting antimalarial drug and an artemisinin derivative are the components of ACT (amodiaquine, lumefantrine, mefloquine, piperaquine or sulfadoxine-pyrimethamine).
As part of the National Program, artesunate (four milligrammes per kilogramme of body weight per day) and sulfadoxine (25 milligrammes per kilogramme of body weight), pyrimethamine (1.25 milligrammes per kilogramme of body weight) [AS+SP] are prescribed on Day 0.
For P. falciparum malaria therapy in northeastern India, the fixed dosage combination (FDC) of Artemether-lumefantrine is now the recommended ACT in the national drug policy, after recent reports of late treatment failures to the existing combination of AS+SP (AL).
Fixed-dose combinations of artesunate-amodiaquine, amodiaquine-mefloquine, and arterolane-piperaquine (for adults only) are registered for marketing in India and can be used to treat uncomplicated P. falciparum or mixed infections, even though the national programme in NE states uses AL and the rest of India AS+SP.
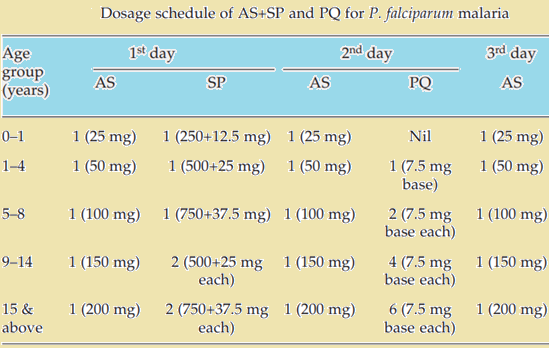
India has prohibited the monotherapy of oral artemisinin derivatives
To date, the only quickly acting antimalarials are derivatives of artemisinin; nevertheless, if taken alone, this may contribute to the development of resistance to artemisinin-based drugs. As a result, they should not be used as a monotherapy for uncomplicated malaria unless in particular studies on artemisinin resistance after consulting with NVBDCP and NIMR or as injectables for severe malaria. Only severe malaria should necessitate the use of injectable artemisinin derivatives.
| Tablet AL |
AL dose total (BD for 3 days) |
Number of tablets in the packing |
Tablets to be given (BD for 3 days) |
|
5-14 kg (>5 months to <3 years) |
20 mg/120 mg | 6 | 1 |
|
15-24 kg (>3 to <9 years) |
40 mg/240 mg | 12 | 2 |
|
15-24 kg (>3 to <9 years) |
60 mg/360 mg | 18 | 3 |
|
>34 kg (<14 years) |
80 mg/480 mg | 24 | 4 |
Management of malaria during pregnancy
Pregnant women with P. falciparum malaria should be treated with ACT in the second and third trimesters, and with quinine in the first trimester. Chloroquine is an effective treatment for malaria caused by Plasmodium vivax.
Management of Infections with multiple strains
P. falciparum infections that are mixed with other parasites should be treated as falciparum malaria. ACTs other than AS+SP should be used in vivax malaria since they are more efficacious. Nevertheless, primaquine may be used as an anti-relapse medication for 14 days if necessary.
Clinical criteria-based treatment without laboratory confirmation
Diagnosing malaria should be done using microscopy or a rapid diagnostic test (RDT). As shown below, however, there are a few exceptions to the rule.
- Patients with a negative P. falciparum RDT should be treated with chloroquine at the full therapeutic dosage of 25 mg/kg body weight over three days if they display signs or symptoms of malaria and there is no evident reason for their fever.
- It's best to finish the therapy according to the species if the slide results come in later.
- If RDT or microscopy cannot establish the presence of malaria in suspected cases, then the full therapeutic dosage of chloroquine should be administered.
General guidelines for treating uncomplicated malaria
- Avoid commencing therapy without food in your stomach.
- Dosage 1 should be administered under medical supervision.
- If vomiting occurs within half an hour after taking antimalarials, the dose should be repeated.
- When symptoms don't improve after 48 hours or worsen, the patient should be asked to come back and report back.
- The patient should also be evaluated and investigated for any concurrent conditions.
Chemoprophylaxis
Chemoprophylaxis is suggested for travellers, migratory workers, and military personnel in malaria-endemic regions. Pregnant women and other susceptible groups should be urged to use personal protective measures like insecticide-treated bed nets.
Short-term chemoprophylaxis (less than 6 weeks)
Adults should take 100 mg of doxycycline daily, while children over the age of 8 should take 1.5 mg/kg of body weight. The medicine should be administered two days before travel and for four weeks after leaving a malarial location. Doxycycline is contraindicated in pregnant women, nursing mothers, and children less than 8 years old.
Long-term chemoprophylaxis (more than 6 weeks)
Mefloquine: 5 mg/kg body weight (up to 250 mg) once a week for two weeks before, during, and after leaving the region. Mefloquine is contraindicated in patients having a history of convulsions, mental disorders, or cardiac disorders.
How to manage severe malaria
In the presence of P. falciparum and no other clear source of symptoms, having one or more of the following clinical or laboratory characteristics implies severe malaria :
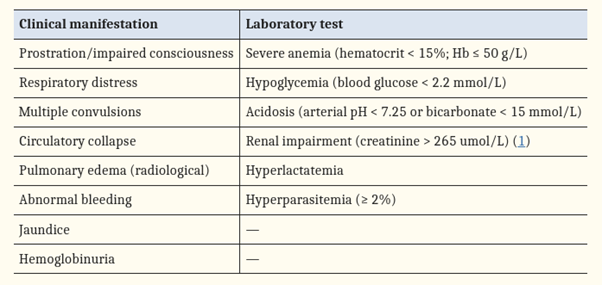
Severe malaria is a life-threatening situation that requires immediate treatment. As a particular antimalarial treatment, artemisinin derivatives of quinine should be administered parenterally. The intravenous method is preferable to the intramuscular approach. Artesunate: 2.4 mg/kg body weight administered intravenously (i.v.) or intramuscularly (i.m.) on admission (time=0), then at 12 and 24 hours, then once daily (Care should be taken to dilute artesunate powder in 5 per cent Sodium bi-carbonate provided in the pack).
On admission, 20 mg quinine salt/kg body weight (i.v. infusion in 5% dextrose/dextrose saline for 4 hours) is given, followed by a maintenance dosage of 10 mg/kg body weight every 8 hours; the infusion rate should not exceed 5 mg/kg body weight per hour. If the patient has previously received quinine, a loading dosage of 20 mg/kg body weight need not be administered. Never administer Bolus Injection of Quinine
If parenteral quinine treatment must be prolonged beyond 48 hours, the dosage should be decreased to 7 mg/kg body weight every eight hours. Arteether: 3.2 mg/kg body weight i.m. on admission, followed by 1.6 mg/kg body weight each day. – Arteether: 150 mg i.m. daily for three days in adults only (not recommended for children). Intravenous formulations are superior to intramuscular preparations. Parental therapy should be administered for a minimum of 24 hours after initiation.
Note: When the patient is able to accept oral medication or after at least 24 hours of parenteral therapy, the subsequent treatment should consist of the following: Patients taking artemisinin derivatives should complete their oral ACT regimen. In cerebral malaria, however, ACTs containing mefloquine should be avoided owing to neuropsychiatric effects. Patients taking parenteral quinine should also get an oral course of ACT. In the first trimester of pregnancy, the medicine of choice is parenteral quinine. If quinine is unavailable, however, artemisinin derivatives may be administered to preserve the mother's life. In the second and third trimesters, artemisinin derivatives administered parenterally are favoured.
An emerging concern of severe malaria due to P. vivax
P. vivax-caused severe malaria has received significant attention in recent years. Several instances have been documented in India, and there is cause to be concerned that this issue may become more prevalent in the future years. Malaria produced by the parasite Plasmodium vivax should be treated similarly to that caused by Plasmodium falciparum; however, primaquine should be administered for 14 days to avoid recurrence when the patient recovers from acute sickness and can take primaquine.
The recent recommendation of malaria vaccine by WHO (2021)
The RTS, S/AS01 malaria vaccine should be administered to prevent P. falciparum malaria in children residing in WHO-defined areas with moderate to high malaria transmission.
- Four doses of the RTS, S/AS01 malaria vaccine should be administered to children beginning at 5 months of age.
- In locations with significant seasonal malaria transmission or perpetual malaria transmission with seasonal peaks, countries may consider administering the RTS, S/AS01 vaccine seasonally using a five-dose regimen.
- Countries that opt to offer the vaccine as part of a five-dose seasonal plan are asked to describe their experiences, including any adverse events that occurred after vaccination.
- Malaria vaccine RTS, S/AS01 should be administered as part of a comprehensive malaria control plan.
Click here to see references
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author is a practising super specialist from New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries