Hypofractionated Radiotherapy: Efficacy and Toxicity Considerations
M3 India Newsdesk May 16, 2024
This article provides an overview of hypofractionated radiotherapy (HFRT) in the context of breast and prostate cancer treatment. It emphasises its radiobiological rationale, clinical trials demonstrating efficacy and safety, as well as considerations for risks and toxicity.
Standard radiotherapy (RT) delivers the total radiation dose, to a target volume, in a series of smaller dose increments, termed fractions.
A typical Conventionally fractionated RT (CFRT) for solid cancers refers to treating with one fraction per day, 5 times a week, during several weeks with a dose per fraction of 1.8-2 Gy, up to a total of 50–80 Gy, although this varies across treatment centres, tumour types and patients [1].
Conventionally, 1.8–2 Gy was chosen based mostly on the severity of early skin reactions and local control rates in squamous cell carcinomas of the head and neck and uterine cervix. This choice was empirically developed in the early 20th century [2].
This traditional fractionation regimen is aimed at maximising local tumour control while minimising toxicity to healthy tissues. However not all tumours may benefit from this, in which case the dose per fraction can be raised without compromising the therapeutic outcome [3].
When the total dose of radiation is divided into large doses and treatments are given once a day or less often, it is called Hypofractionated RT (HFRT) or hypofractionation. So, Hypofractionation is defined as >2 Gy/fraction; with moderate hypofractionation defined as >2 to <5 Gy/fraction and ultra hypofractionation as≥5 Gy/fraction. These thresholds are biologically arbitrary but are useful in the discussion of clinical trials. HFRT is given over a shorter period than standard RT[4].
Hypofractionation is an older concept and a moderately hypofractionated schedule for laryngeal cancer (50 Gy/16 fractions) was developed in Manchester during the 1930s and is still in use today.
With the passage of time, there has been heterogeneous use of hypofractionation at various centres [4].
A 1989 UK survey revealed dozens of different regimens used for common radical and palliative indications [5].
Radiobiological rationale for hypofractionation
A successful radiation treatment plan combines local tumour suppression with little late normal tissue effects. These can be quantified using the tumour control probability (TCP) and normal tissue complication probability (NTCP) [6].
While most types of cancer benefit in terms of disease control and toxicity from CFRT schedules, some cancers have shown to be more sensitive to fractional doses [7].
This heterogeneity in terms of biological effects with CFRT can be explained by the linear quadratic (LQ) model, which describes the cell survival (CS) curves as a function of radiation dose [8].
It is traditionally accepted that radiation injury takes place inside the DNA through two types of damages: alpha (α) type, which is non-repairable and happens when there is a double-strand break of DNA caused by the passage of a single charged particle e.g. electron, proton, heavy ion, and beta (β) type, which is repairable with time and is due to two separate single-strand breaks caused by different charged particles [9].
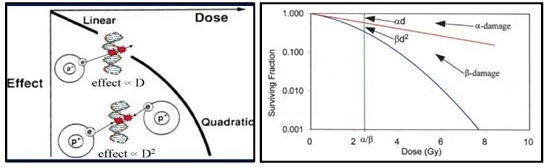
Figure-1: (a) Linear-quadratic Model; (b) Linear-quadratic cell survival curve, with α = 0.22 Gy-1 and α/β = 2.5 Gy (Log cell kill for α-type damage equals that for β-damage at a dose equal to α/β)
In the LQ model, α is the initial inclination of the CS bend and illustrates the intrinsic sensitivity of the cell to radiation. This coefficient is linearly dependent on the dose. In addition, β is the curvature of the subsequent segment of the plot and is related to the dose-per-fraction and dose rate variability.
This coefficient is proportional to the square of the dose, which leads to the model being quadratic [Figure-1(a)]. As a result, a higher α/β ratio is represented by a more linear CS curve, whereas a lower α/β ratio is related to a more inclined CS curve [10].
In practical terms, this ratio defines the fraction dose where both the alpha and beta components cause the same value of cell death [Figure-1(b)]. Therefore, early-responding tissues or tumours with a rapid turnover are characterised by a high α/β ratio and are less sensitive to fraction size or dose rate variations, while late-responding tissues or tumours with a slow turnover are characterised by low α/β ratio and are more susceptible to fraction size or dose rate increments [11].
Based on the above, it should be noted that the advantage of hypofractionation depends on the α/β values of the target/tumour about the surrounding normal tissues [12].
Breast & prostate cancer
RT is usually used in breast cancer in the adjuvant setting, to a lower dose than radical doses given at other tumour sites. The purpose of treatment is to eliminate any residual microscopic disease after surgery. On the other hand, external beam radiation or brachytherapy is used to administer a greater radical dose during prostate radiation therapy [13, 14].
The α/β ratios of breast and prostate cancers are the same or less than the surrounding late-reacting tissues. These low α/β ratios reflect their slower proliferation rates compared with other tumour types, so, they respond similarly to late-responding normal tissues.
Thus, HFRT schedules are primarily attractive in the treatment of breast and prostate cancers, which improve therapeutic index. Hypofractionation can achieve this in one of two ways when compared with CFRT:
- Dose escalation to increase tumour control while maintaining the same NTCP (i.e. shift the TCP curve to the left-Figure-2)
- Maintaining dose equivalence regarding tumour cure probability while decreasing the normal tissue dose (i.e. shifting the NTCP curve to the right-Figure-2[13].
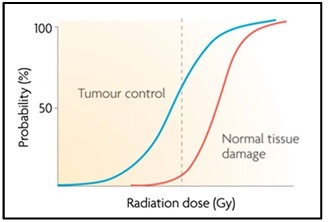
Figure 2: Sigmoid-shaped curves for tumour control (TCP, left) and normal tissue complication/damage (NTCP, right) probability. The dashed lines indicate a 60% TCP and a 5% NTCP for a given dose[6]
Modern RT techniques like Volumetric Modulated Radiotherapy (VMAT), Intensity Modulated Radiotherapy (IMRT), 3-dimensional Conformal Radiotherapy (3D-CRT), etc. have been used in CFRT to decrease the normal tissue dose and their use also minimises the toxicity of hypofractionated radiotherapy. Other advantages of HFRT are conferred in terms of logistical, patient convenience and resource allocation considerations [14].
Reduced numbers of fractions reduce radiotherapy costs in terms of work hours and fewer fractions also result in fewer visits for a patient to the radiotherapy centre, which is more convenient and less costly [15].
These benefits, however, ought to be viewed as "added bonuses" to a hypofractionated schedule, since the main objective of the latter should be therapeutic benefit. But, even if HFRT is shown to be no more toxic than CFRT with similar efficacy, it can be argued that the logistical advantages are sufficient to warrant clinical implementation.
Breast cancer
- One of the first hypofractionation trials in breast cancer was done in the UK in the 1986-Standardisation of Breast Radiotherapy pilot trial (STARTpilot; n = 1410) and in Canada in 1993 with the Ontario trial (n = 1234 [16].
- These were followed in 1999 with the START-A and START-B trials [17]. These trials challenged the conventional wisdom that cancers in the breast are less sensitive to changes in a dose per fraction than the dose-limiting surrounding normal tissues, which justified the rationale for 50 Gy in 25 fractions.
- They conducted a comparison between 50 Gy in 25 fractions with 41.6 Gy or 39 Gy in 13 fractions over 5 weeks in START-A (n = 2236) and with 40 Gy in 15 fractions over 3 weeks in START-B (n = 2215), the latter of which is already widely used in the UK, to test the hypothesis that breast tumour tissue and surrounding late-reacting normal tissue are similarly sensitive to fraction size.
- The START-pilot and START-A trial were designed to allow estimation of the α/β ratios of breast tumour and late responding normal tissue and similar α/β ratios were found for breast tumour using locoregional relapse as the endpoint and surrounding normal tissue in the range 3.5–4.7 Gy.
- Ten-year follow-up data found that breast shrinkage, telangiectasia and breast oedema were significantly less frequent with 40 Gy than 50 Gy in START-B, with no evidence that 40 Gy was less successful in obtaining locoregional control in 15 fractions. These trials resulted in the standard breast cancer radiotherapy treatment protocol in the UK changing to 40 Gy in 15 fractions[17].
- The randomised UK FAST trial (2004–2007, n=915) went further with its hypofractionation regimen, testing 50 Gy in 25 fractions over 5 weeks against either 30 or 28.5 Gy in five fractions over 5 weeks. After a 3-year median follow-up, the 28.5 Gy scheme was comparable with the 50 Gy scheme in terms of adverse reactions, and lower than the 30 Gy scheme because the total dose to the tissue was lower [18].
- This trial was followed by the FAST Forward trial, which closed 2 years earlier than expected after very rapidly recruiting 4000 patients. In terms of 5-year ipsilateral local tumour relapse to 40 Gy in 15 fractions, FAST Forward demonstrated that 26 Gy in five fractions of 5.2 Gy to the preserved breast or post-mastectomy chest wall following primary surgery was non-inferior.
- The 5-day schedule caused milder early skin reactions and similar rates of late adverse effects and was as effective and safe as the standard 15-fraction regimen after primary surgery for early breast cancer with major benefits of convenience and cost for patients and health services globally [19].
Prostate cancer
- The most relevant study on moderate hypofractionation for localised prostate cancer is the CHHiP study, a randomised phase-III trial, which recruited 3216 patients with localised prostate cancer (pT1b–T3aN0M0).
- In this trial, all patients were allocated (1:1:1) to receive either CFRT (74 Gy/37 fractions) or one of two hypofractionated schemes (either 60 Gy/20 fractions or 57 Gy/19 fractions). At 5 years, the incidence of ≥grade 2 bowel, bladder and sexual-clinician-reported toxicities were similar in all the groups of treatment.
- So, the integration of hypofractionation with high-quality treatment methods offers outstanding tumour control, lower toxicities and greater benefits for men with prostate cancer compared to CFRT[20].
- Similarly, The PROFIT trial, which enrolled 1204 patients with intermediate-risk disease to receive either a treatment of 78 Gy in 39 fractions or 60 Gy in 20 fractions, showed that both regimens had similar biochemical and clinical failure rates (85% in both arms) at 6 years, with lower late toxicity rates with HFRT [21].
- In the RTOG 0415 trial (n=1115; low-risk disease), patients received either 73.8 Gy in 41 fractions or 70 Gy in 28 fractions. Final results demonstrated noninferiority of 70 Gy in 28 fractions in terms of efficacy but with greater grades 2 and 3 gastrointestinal and genitourinary late toxicities [22].
- Based on the above, there is a solid consensus to perform moderate hypofractionation in patients with prostate cancer in all risk groups. Schedules of 60 Gy in 20 fractions and 70 Gy in 28 fractions are supported by the broadest body of evidence, even in the event that an ideal regimen cannot be identified.
Risks & toxicity considerations associated with hypofractionation in breast and prostate cancer
To some extent, the hypofractionation approach is less toxic in breast cancer than in prostate cancer, as for the former lower relative doses are used in the adjuvant rather than radical setting.
The HFRT schedules used in breast and prostate cancer are based on the assumption that the α/β ratio is lower than that of the surrounding normal tissue. Although measurements of α/β have been carried out using clinical data, these represent population averages and suffer from very large 95%-confidence intervals reflecting large inter-patient variability[17,23].
The START-A breast cancer hypofractionation trial recruited 2236 women but still yielded notably large 95% confidence intervals[17].
One prostate hypofractionation trial, which used a combination of external beam radiotherapy with high-dose-rate brachytherapy delivered in two or three implants, measured an estimated α/β of 1.2. However, because only 192 patients were recruited for their trial, the 95% confidence interval on this value was 0.03–4.1 Gy [23].
The inter-patient variability of α/β values suggests that not all patients benefit from the same hypofractionated schedule and may have higher toxicity rates, which could be a result of many factors, including insufficient time for normal tissue to repair between fractions and the prescribed dose being delivered to too large a volume[24].
Like any radiation treatment, a hypofractionated schedule might cause secondary malignancies and unduly increase normal tissue problems if the α/β ratios are not precisely predicted and the doses are not chosen appropriately.
One study comparing the late-responding normal tissue complications in prostate cancer radiotherapy discovered that a hypofractionated radiation schedule resulted in a higher frequency of complications for patients with initial urinary tract issues than a conventional schedule [25].
Future steps in the clinical implementation of hypofractionated schedules should involve the development of biomarkers to measure individual α/β ratios for each patient, while factoring in tumour heterogeneity, to enable prediction of the response to a particular treatment regimen, ultimately personalising the radiotherapy schedule to maximise the therapeutic index and obtain optimum outcome [3].
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Pankaj Kumar is a Director at the Department of Radiation Oncology, at Max Super Speciality Hospital, Mohali, Punjab.
Co-Author: Dr Rupali Aggarwal is a Senior Consultant, at the Department of Radiation Oncology, Sohana Hospital, Mohali, Punjab.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries