How and when to use steroids in Rheumatoid Arthritis: Dr. Rohini Handa
M3 India Newsdesk Jul 01, 2020
Dr. Rohini Handa explains the role of low-dose and high-dose steroids in Rheumatoid Arthritis (RA), types of corticosteroids and when to use them in established RA, and useful facts for clinicians to use steroids in resource-constrained settings.

Rheumatoid Arthritis (RA) is the most common inflammatory polyarthritis chanced upon in clinical practice. The use of corticosteroids in the treatment of autoimmune rheumatic diseases is all pervasive. RA is no exception. Steroids are widely used, and equally widely misused, in the treatment of RA.
Be it a rheumatologist or an internist, be it an inexperienced young resident or a distinguished old clinician, be it a thinker at the cerebral level or a doer at the spinal level, the topic of steroids is likely to evoke a spirited or opinionated response from doctors. This piece summarises the current role of steroids, or more correctly glucocorticoids, in the treatment of RA.
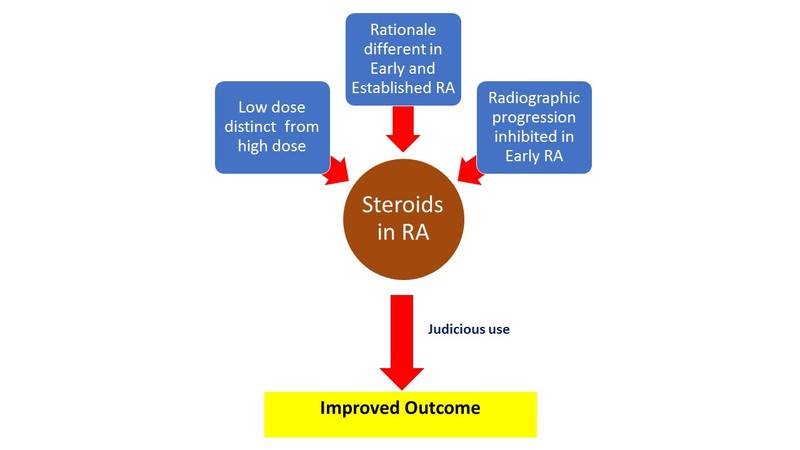
Fact file for the clinician
- Not all patients with RA need steroids. Not all patients on steroids need them all the time. When indicated, low doses are employed for the shortest time.
- In newly diagnosed, early RA (<6 months), steroids are used as ‘bridge therapy’ and to inhibit radiographic progression. The latter benefit is over and above that conferred by DMARDs.
- Steroids should always be used in combination with other DMARDs like methotrexate and never as the sole disease modifying drugs in RA.
- Steroids lower disease activity in established RA and can be used to treat disease flares and as ‘bridge therapy’ when instituting or changing DMARDs.
- Low-dose steroids are safe in pregnant women and breastfeeding mothers.
- Steroids are useful in treatment of several extra-articular manifestations of RA like vasculitis, interstitial lung disease etc.
- In refractory RA, low-dose steroids may improve quality of life dramatically and make a difference between ‘existing’ and ‘living’.
- Judiciously used steroids are perhaps better than injudiciously (and sporadically) employed biologics.
- Despite the favorable risk benefit ratio of low-dose steroids, no dose is absolutely safe.
- Methyl prednisolone and deflazacort have no added benefits over inexpensive prednisolone.
How common is steroid use in RA and why?
Current corticosteroid use is reported by as many as one-third of patients while exposure any time is reported by more than two-thirds of the patients. What makes patients and doctors reach out for steroids?
For one, steroids rapidly and effectively relieve the pain of RA. Pain has been dubbed as the 5th vital sign. Behind the razzmatazz of immune-biology, cytokines and inflammatory pathways, patients and their families have one desire- to get succour from pain.
Easy availability, low cost, rapid onset of action, marked abrogation of inflammation with dramatic short term results—and you don’t need to be an Einstein to fathom why these drugs are still around more than 70 years after they were first introduced to treat RA.
The fall and resurrection of steroids in RA
The frontline drugs to treat RA include non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, disease modifying anti-rheumatic drugs (DMARDs) and biologics. The discovery of cortisone was a major triumph in the fight against RA which culminated in one of the fastest awards in the venerable history of the Nobel prize- discovery in 1948 and the bestowment of Nobel in 1950.
Showcased as a cure for RA, the large dosages used and the consequent side effects turned euphoria into disenchantment. From being touted as heroes, they were soon branded as villains and the use declined. This, coupled with the emergence of DMARDs like gold, sulfasalazine, methotrexate, hydroxychloroquine, etc., relegated steroids to the background in RA.
A reappraisal of steroid therapy was accelerated by the publication of The Arthritis and Rheumatism Low Dose Glucocorticoid Study Group results in 1995. [1] This paper, despite its shortcomings, drew attention to the fact that steroids had the potential to retard radiographic progression in RA. This led to a paradigm shift in the thinking about the role of steroids: not only were they effective in reducing inflammation, they also had disease modification potential. Pari passu with benefit on radiologic damage, investigators began to talk about the difference between low-dose and high-dose steroids and early versus established RA.
Does dose matter- The concept of low-dose steroids
It is now believed that the side effect profile of ‘low-dose’ corticosteroids as used in RA is very different from ‘high-dose’ steroids as used in some other conditions. Low-dose steroids is a daily prednisolone dose of 7.5 mg or less. Medium dose refers to prednisolone dose between 7.5-30 mg daily while high dose is >30 mg per day. The dose advocated in RA is usually <7.5 mg/day. It could be as low as 2.5 mg daily or even on alternate days and needs to be individualised.
The concept of early versus established RA
The cut off between early and established RA is a moving target and contemporary studies have progressively whittled this duration from 3 years through 1 year to the current 6 months. The rationale for steroid use in early RA is very different from use in established disease.
Steroid use in early RA
The addition of low-dose prednisolone to DMARDs like methotrexate leads to a reduction in radiographic progression. Such a use to harness the disease modifying potential of steroids is advocated only in early RA with the caveat that steroids should never be used as monotherapeutic agents but always combined with DMARDs like methotrexate. The retardation of radiographic damage is over and above that achieved by DMARDs alone. While an earlier Cochrane review [2] talked about such a use for 1-2 years, the optimum duration to capitalise on the radiographic inhibition is not known.
When to use steroids in established RA
Corticosteroids in established RA are used in the following situations:
- As ‘bridge therapy’ at the time of institution of DMARDs like methotrexate. DMARDs take several weeks to show their effects. Corticosteroids bridge the gap between institution of DMARDs and onset of action. This use is for 10 to 12 weeks.
- Pregnancy and lactation: Low-dose prednisolone is safe during pregnancy and lactation. DMARDs that are safe during pregnancy and lactation include hydroxychloroquine and sulfasalazine.
- Disease flares when corticosteroids may be used for a few weeks to suppress disease activity.
- Patients with refractory disease may require low-dose corticosteroids to maintain an acceptable quality of life.
- RA with extra-articular manifestation like interstitial lung disease, vasculitis, mononeuritis multiplex etc. may require corticosteroids, sometimes in higher dosages.
Most of the aforementioned conditions require low-dose corticosteroids. Some conditions like vasculitis may require higher dosages for initial disease control. Eye inflammation may require topical corticosteroids while the odd, recalcitrant joint that is active in face of globally quiescent disease can be injected with intra-articular steroids rather than hiking systemic treatment.
Steroids in resource-constrained settings
The advent of biologics, and now the Janus kinase (JAK) inhibitors, has dwarfed the role of steroids. Current European League against Rheumatism (EULAR) guidelines recommend steroid discontinuation in about 3 months. Patients failing to achieve disease control in this time frame are offered biologics or JAK inhibitors. In our country, where the biologic penetration is low and the average biologic use is 6 months because of costs, this is a utopian dream.
It has been shown that combination-DMARD treatment, especially when combined with periodic glucocorticoids, may be as effective as a biologic agent plus methotrexate. [3] This fact can be leveraged to the advantage of patients who cannot afford biologics or JAK inhibitors.
Will this paradigm change in the future?
The availability of low-cost, generic JAK inhibitors in the near future is likely to change the whole equation. Biologics and biosimilars are expensive, given the complexities of manufacture. However, JAK inhibitors like tofacitinib and baricitinib are not as difficult or expensive to produce. Currently, they are priced equivalent to the biologics. With the entry of generics in the Indian market, future treatment algorithms of RA are likely to see methotrexate plus JAK inhibitor combinations overtake the use of steroids. The issue is not about the efficacy of steroids. It is more to do with the adverse effects that in the long term, even in low doses, are perceived to be more than biologics or JAK inhibitors.
Are low-dose steroids bereft of side effects?
Low dose does not confer exemption from steroid side effects. No dose is absolutely safe. Due diligence must be exercised as with any other drugs. Thus, strategies to minimise complications like bone protective agents etc. should be instituted concurrently with the steroids. The lowest dose for the shortest time should be employed. Prolonged use, even of low doses, carries with it the risk of all the well known side effects of steroids.
Type of cortoicosteroids- Does it matter?
Prednisolone, methylprednisolone, betamethasone, dexamethasone, and deflazacort are the corticosteroids used in clinical practice. Long-acting steroids like dexamethasone are best avoided. So far as oral use is concerned, there is no robust evidence to suggest that methylprednisolone and deflazocort confer any advantage over simple prednisolone.
Corticosteroids in Rheumatoid Arthritis: Status 2020
Sequence of drug withdrawal in RA
Once sustained remission is achieved in RA, treatment can be de-escalated. In a patient who is on steroids plus DMARDs, corticosteroids should be tapered and stopped first. Conventional DMARDs like methotrexate are continued virtually indefinitely, albeit in lower doses.
Conclusions
Corticosteroids are invaluable in the treatment of RA. The drugs are good, their indiscriminate use is bad. It is for the clinician to balance the dichotomy of proven efficacy and some side effects while individualising treatment in the disease that is RA.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Rohini Handa is a Senior Consultant Rheumatologist from Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries