Case 2: A delicate sound of thunder: Diagnosis of PE: What was the treatment approach?- Part 4
M3 India Newsdesk Dec 28, 2020
Ready for the big reveal? Prof. Dr. Sundeep Mishra provides answers to the questions from the previous part and divulges details of how the final diagnosis was made and the treatment that followed for the patient.
Click to read other articles from Dr. Sundeep Mishra.
Question 1: Are any more investigations required?
Echocardiography
Echocardiography has a low positive and negative predictive value (≤50%), and expected echocardiography signs of RV overload and RV dysfunction are non-specific for pulmonary embolism. The only finding of some discriminative (positive predictive) can be impaired RV ejection pattern or decreased contractility of the RV free wall compared with the RV apex. However, finding of RV dilatation (present in ~1/4th) is co-relative of poor prognosis. Thus, echocardiography can be an additional investigation but cannot be a substitute for CTPA.
Findings of Doppler Tissue Imaging and wall strain are also non-specific. However, echocardiography may be useful in differential diagnosis of the cause of shock; pericardial tamponade, acute valvular dysfunction, severe global or regional LV dysfunction, aortic dissection, or hypovolemia. On the other hand, one area where echocardiography can help is visualisation of right heart thrombi which should be sufficient to establish diagnosis in conjunction with RV dysfunction. Transesophageal echocardiography may also be useful to identify central pulmonary embolism.
Magnetic resonance angiography
Gadolinium enhanced magnetic resonance angiography (MRA) is a relative new imaging technique, introduced to do away with major drawbacks of CTPA; radiation exposure, enhancement of iodinated contrast. Using standard/gated spin-echo techniques, pulmonary emboli demonstrate increased signal intensity within the pulmonary artery. Compared to gold-standard pulmonary angiography sensitivity of the test is variable (77-100%) but specificity is high (95-99%). However, one problem with MRA is that in some cases MRA may be non-interpretable; 2/3rd due to non-opacification of segmental/sub-segmental branches (67%) and 1/3rd due to motion artifacts. Another major problem is longer acquisition time. Thus, in clinical practice value of MRA (even if it is available) is limited.
DVT work-up
- Ultrasound for lower-limbs, iliac vein etc.
- Work-up for Coagulopathy – APT, INR, protein C, protein S, and antithrombin III are other risk factors which can be estimated
Work up is important and can be carried out once the patient gets stabilised, but at the moment, immediate is most important.
Serial ECG
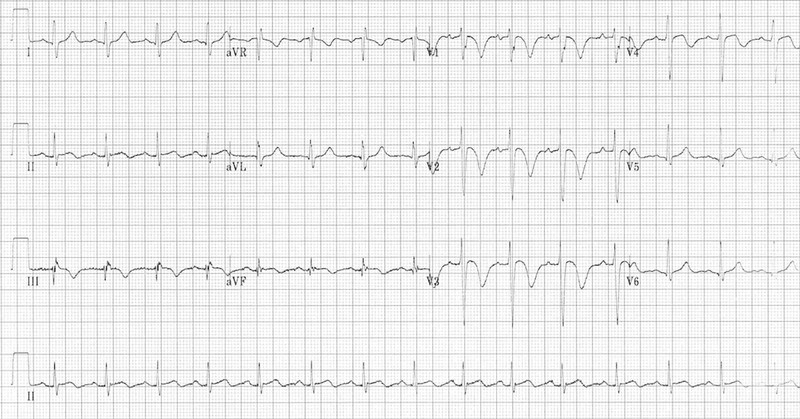
Important in case of PE in patients: First ECG below had revealed sinus tachycardia, T-wave inversions in the anterior (V1-4) and inferior leads (II, III, aVF) and possible ST elevation in III and aVF. On the face of it, the ECG seemed consistent with diagnosis of ACS but reality is that negative T waves in leads III and V1 are very uncommon in a case of ACS (observed in only 1% of patients with ACS) but is rather consistent with pulmonary embolism (occur in nearly 9 out of 10 cases of pulmonary embolism). Moreover, ACS is rarely associated with tachycardia.
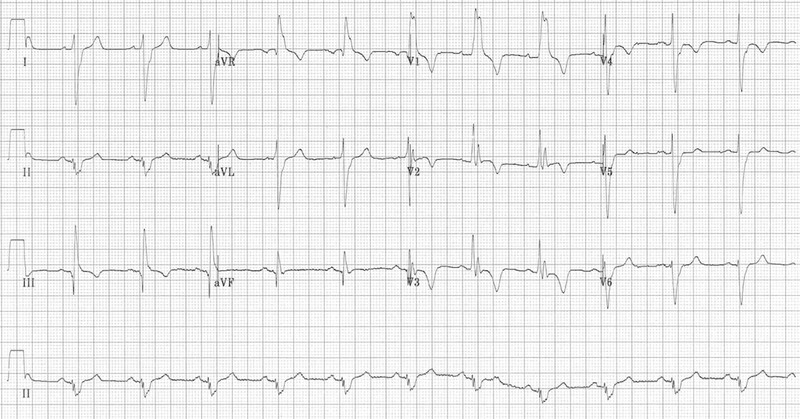
The second ECG, however, was far more representative of a case of pulmonary embolism and perhaps represents a progression of disease (below). RBBB, extreme right axis deviation (+180 degrees), S1 Q3 T3, T-wave inversions in V1-4 and lead III and clockwise rotation with persistent S wave in V6.
S1Q3T3 is considered classical of PE but is not the commonest finding (< 1 in 5 have these findings). Acute T wave inversion in anterior leads and RBBB (complete/incomplete) suggests right ventricular overload due to choked pulmonary circulation. The findings of right ventricular overload can resolve quickly after pulmonary circulation is restored as a result of optimal therapy.
Natriuretic peptides
Brain natriuretic peptide (BNP) tests are neither sensitive nor specific for pulmonary embolism but higher levels do have a prognostic value. It has been reported that BNP level >100 pg/mL or an NT-proBNP level >600 ng/L had an all-cause in-hospital mortality rate 6- and 16-fold higher.
Our patient had NT-proBNP level 534 pg·mL.
Question 2: What is prognosis of this patient with massive PE and shock?
Among patients who develop PE, ~10% die within the first hour, and 30% die subsequently from recurrent embolism. if left untreated. On the other hand with prompt anti-coagulation mortality is <5%. Regarding V/Q mismatch, >1/3rd of defects are resolved in 2 weeks, >1/2 defects within 3 months, overall nearly 3/4th defects become normal on long term.
Mortality with massive pulmonary embolism ranges between 30-60% despite treatment, but when it is associated with shock (as in our case), it can be even higher.
- ↑plasma levels of natriuretic peptides (brain natriuretic peptide and N -terminal pro-brain natriuretic peptide >500 ng/L )
- ↑plasma lactate levels (≥2 mmol/L) are associated with ↑risk of mortality and other adverse outcomes
Shock/hypotension and evidence of right ventricular overload are also other factors co-related with poor prognosis. Our case was of massive pulmonary embolism with evidence of shock and RV overload who had already witnessed cardiac collapse and on IABP.
Question 3: How do we treat now?
Risk stratification
All patients with PE require rapid risk stratification. Our case belongs to high-risk category because of presence of massive pulmonary embolism and associated shock, evidence of RV overload and even mild hypoxemia.
Anticoagulation
All patients require some form of anticoagulation; unfractionated heparin (UFH), low molecular weight heparin (LMWH), fondaparinux. However, in high-risk cases when thrombolysis, interventional or surgical therapy is planned IV UFH is preferred over LMWH or fondaparinux.
In our case since angiography ± angioplasty was planned; patient was already given 5000 U of IV UFH, followed by infusion at the rate of 1000 U/hr.
Thrombolysis
Patients presenting with hypotension (systolic BP <90 mmHg) who do not have a high bleeding risk can receive thrombolytic therapy as soon as the diagnosis is made and it can reduce mortality (reduced mortality by ~½ but associated with a 2.7-fold increase in major bleeding especially in older individuals). In elderly patients (>65 years there was x3 times the risk of bleeding).
Our patient was an elderly lady (79 years) and therefore much higher risk of bleeding and therefore thrombolysis is not the preferred option. Moreover, we have a round-the-clock cath lab facility so catheter intervention was planned.
Catheter embolectomy
Catheter embolectomy and fragmentation is reasonable for patients with massive pulmonary embolism especially if they have clinical evidence of an adverse prognosis:
- New haemodynamic instability
- Worsening respiratory failure
- Severe right ventricular dysfunction
- Evidence of major myocardial necrosis (elevated troponins or cardiac markers) and with high bleeding risk
Practically all these factors were present in our patient. In addition, the patient had already witnessed cardiac collapse and was on IABP support, so she was taken for catheter-based embolectomy/thrombolysis. As a matter of fact, the patient should have undergone pulmonary angiogram the moment coronary angiography turned out normal, but this opportunity was missed!
Pulmonary artery angiography showed significant and severe emboli in bilateral branches of the pulmonary artery. Catheter-directed thrombolysis with urokinase was administered.
Surgical embolectomy
Surgical pulmonary embolectomy can be performed when catheter intervention fails or as an alternate to catheter intervention in suitable cases. However, in our case, catheter-based thrombolysis was successful as evidenced by future course.
What happened next?
The clinical condition improved remarkably and serial echocardiography revealed gradual recovery of heart contractility (LVEF improved from 25% to 55% in one week. The patient was weaned off IABP after 4 days and was discharged from hospital in a stable condition after 14 days.
Final discussion
The clinical presentation of pulmonary embolism is fairly ambiguous, making it difficult to propose an exact diagnosis at an early stage. In our case focus on possible ST segment elevation in exclusion to other findings was a mistake. Coronary angiography revealed no obstructive lesions and diagnosis was only established with CT pulmonary angiography. Later, invasive (catheterisation) pulmonary angiography revealed near-total occlusion of the right pulmonary artery.
The patient was treated with catheter-based thrombolysis despite initial poor prognosis. It is possible that timely IABP institution could have been the savior; providing enough cardiac assistance and haemodynamic stability in the interim period until definitive treatment could be provided.
Take home messages
- Pulmonary embolism is a common disease with incidence of around 1 case per 1000 persons per year. It is the second-most common cause of sudden death and third-most common cause of death in hospitalised patients.
- Suspicion of PE is the most important factor in diagnosis although there are no specific signs or symptoms; rather it is a diagnosis of exclusion.
- ECG- Sinus tachycardia and non-specific ST-T changes is the most common abnormality but classic S1Q3T3 pattern occurs only in a minority of cases (<20%). Negative T waves in leads III and V1 and presence of tachycardia help to distinguish it from ACS.
- X-ray is also generally non-specific but rarely more specific signs like Hampton’s hump, Westermark sign or Fleischner sign may be present.
- Diagnostic tests required depend on probability of PE based on clinical scoring like Geneva score.
- Cardiac enzymes or D-dimer tests are generally non-specific but can help in clarifying the probability of PE.
- If pre-test probability is high, CT pulmonary angiogram must be performed to rule in/rule out diagnosis of PE.
- Mortality of PE in sub-massive PE is high (30%) without treatment but with anticoagulation it decreases to <5%.
- With massive embolism or in presence of high-risk features mortality can approach even 60% and more with treatment. High risk features are:
- Hypotension/shock
- Evidence of RV overload (ECG or Echo)
- Hypoxemia
- Elevated BNP level (>100 pg/mL), NT-proBNP level (>600 ng/L) or serum lactatae level
- For massive embolism or in presence of high-risk features thrombolysis, catheter-based embolectomy/thrombolysis or surgical embolectomy may be required.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Sundeep Mishra is a Professor of Cardiology.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries