The Indian Council of Medical Research has started a multi-centre clinical trial to assess the safety of the convalescent plasma to limit any coronavirus related complications.
For our comprehensive coverage and latest updates on COVID-19 click here.
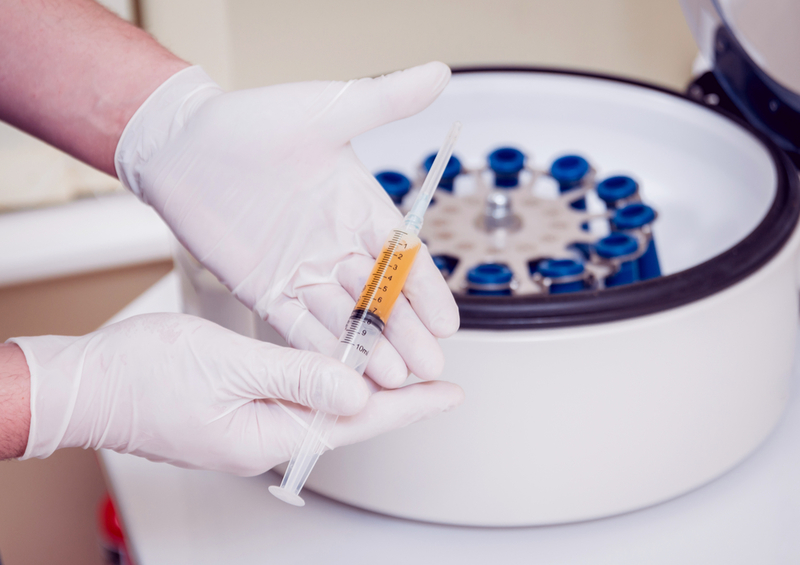 "ICMR has initiated a multi-centre clinical trial to assess the safety and efficacy of convalescent plasma to limit COVID-19 associated complications in moderate disease," read an official statement by the ICMR. The trial has been titled as "A Phase II, Open Label, Randomized Controlled Trial to Assess the Safety and Efficacy of Convalescent Plasma to Limit COVID-19 Associated Complications in Moderate Disease."
"ICMR has initiated a multi-centre clinical trial to assess the safety and efficacy of convalescent plasma to limit COVID-19 associated complications in moderate disease," read an official statement by the ICMR. The trial has been titled as "A Phase II, Open Label, Randomized Controlled Trial to Assess the Safety and Efficacy of Convalescent Plasma to Limit COVID-19 Associated Complications in Moderate Disease."
On April 28, the ICMR had said that currently there are no approved, definitive therapies for COVID-19. Convalescent plasma is one of several emerging therapies. However, there is no robust evidence to support it for routine therapy, the research body said. Meanwhile, the total number of coronavirus cases in the country has reached 49,391, including 14,183 recovered/migrated and 1,694 deaths, as per the Union Ministry of Health and Family Welfare.