Encouraging results for dual-targeted CAR T-cell therapy in hard-to-treat multiple myeloma
American Society of Hematology Annual Meeting Press Release Dec 12, 2019
More than three out of four patients with multiple myeloma that returned or did not respond to at least two therapies remained in remission seven months after treatment with a novel CAR T-cell therapy targeting two proteins that are frequently found on myeloma cells. Those experiencing sustained remissions include nine patients with a difficult-to-treat form of multiple myeloma in which the disease has spread beyond the bone marrow.
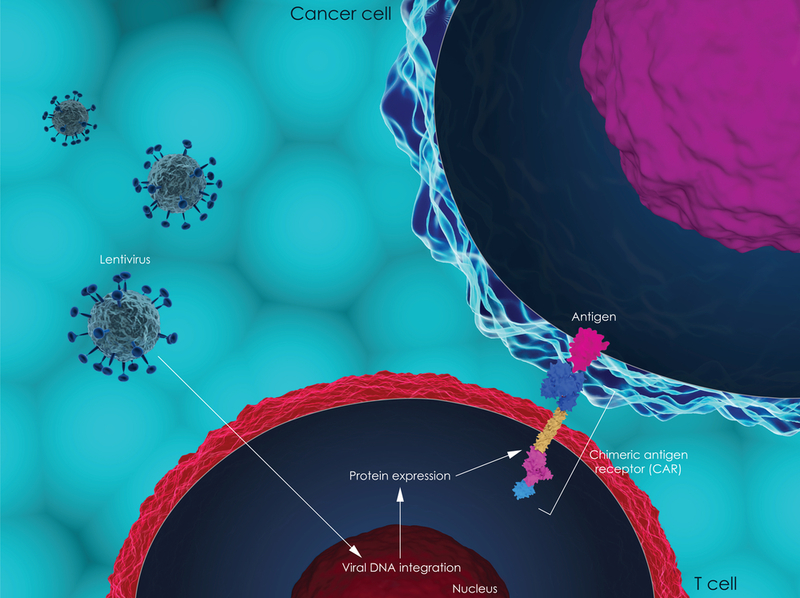
Roughly one in 10 patients with multiple myeloma develop tumors in the organs or soft tissues such as the blood vessels, muscles, and nerves. These so-called extramedullary tumors respond poorly to treatment, and patients who develop them have a poor outlook and poor quality of life, said study author Yu Hu, MD, PhD, of Union Hospital, Huazhong University of Science and Technology in Wuhan, China.
“Our results show that this CAR T-cell product can effectively achieve elimination of extramedullary tumors,” said Dr. Hu. “Although these are preliminary data, they are encouraging for patients with multiple myeloma who have not responded to other therapies.”
The study treatment is the first CAR T-cell therapy to be genetically engineered to target BCMA and CD38, two proteins found on the surface of plasma cells. Multiple myeloma is a cancer of plasma cells, which are found in the bone marrow and are part of the immune system, the body’s defense system against infection and disease.
“Our thinking was that targeting both of these proteins would improve treatment efficacy without increasing toxicity, and induce deeper, more durable remissions,” said Dr. Hu.
The first-in-humans phase I trial enrolled 22 patients whose average age was 59, of whom 11 were men. All had multiple myeloma that had returned or not responded to at least three therapies. Nine of the 22 patients had extramedullary tumors. The study aims were to determine the safest and most effective dose of the CAR T-cell therapy as well as to initially evaluate its effectiveness.
Patients received three days of chemotherapy to “make room” in their immune systems for the engineered T cells. Then each patient was infused with the dual-targeted CAR T cells. Patients were divided into five groups, with each group receiving a higher dose than the previous one. Depending on the cell dose, patients received either one or two infusions.
At a median of 36 weeks of follow-up, 18 patients (90.9%) had MRD-negative disease. Twelve patients (54.5%) had a stringent complete response, meaning that no plasma cells were detected in the bone marrow. Seven patients (31.8%) had a good or very good partial response, meaning that the level of M-protein (an abnormal protein produced by cancerous plasma cells) in the blood or urine was reduced but still detectable. In eight of the nine patients with extramedullary lesions, these tumors were undetectable on their computed tomography scans. For the 17 patients who remained in remission at seven months after treatment, the median duration of response was 28.8 weeks.
Researchers observed some side effects: 20 patients experienced CRS, of whom six needed treatment. No serious adverse neurologic effects such as seizures, movement impairment, difficulty speaking or understanding speech, or fatal swelling in the brain were reported.
“With this dual-targeted CAR T-cell therapy, we have demonstrated a high response rate, especially a higher rate and longer duration of stringent complete response, compared with other therapies, as well as effective elimination of extramedullary lesions, with no serious neurologic adverse effects and manageable levels of other adverse effects,” said Dr. Hu.
The investigators will continue to follow the patients for two years. They are also planning to conduct a phase II trial in both China and the United States to test the treatment’s effectiveness in a larger number of patients.
This study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project fund of Hubei Province of China, and Cellyan Therapeutics Co., Ltd.
This article is a news release from American Society of Hematology Annual Press Meeting. Read the original here.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries