What is Immunotherapy; who shall benefit from it? Dr. Anurag Mehta & Dr. Garima Gupta
M3 India Newsdesk Feb 08, 2019
Immunotherapy has been touted as the future of cancer therapy, but to use it to advantage, it is important to understand the cancer immunity cycle, so as to know who can benefit from it.

Immunotherapy has made deep inroads into the arena of cancer therapy. It has been conferred with encomiums like the ‘future of cancer therapy’, ‘breakthrough in cancer therapy’, ‘jackpot of cancer therapy’ and several others. This enthusiasm has been further given fillip by the 2018 Nobel Prize being awarded to two immunologists, Dr. T Honjo for describing PD1-PDL1 checkpoint inhibition and Dr. James Allison for CTLA4-B7 checkpoint axis.
All of this excitement is not without reason, as Immunotherapy has provided Durable Clinical Benefit (DCB) in some cancer patients including those who have been refractory to several lines of chemotherapy.
With these benefits in mind two questions are raised;
What is Immunotherapy? Who shall benefit from it?
Cancer can be considered as an aggregate of ‘genetic chaos’ and a ‘somnolent immune system’. The genetic chaos drives the transformation of a normal cell to transformed cell with increasingly aggressive attributes garnered through increasing genetic mess.
The immune system normally scans continuously for these rogue cells identified by stress-related antigens expressed by the transformed cells, unique cancer antigens and cryptic antigens normally expressed only at sanctuary sites.
This continuous monitoring and removal of transformed cells is the phenomenon of Immunosurveillance. Subversion of Immunosurveillance allows cancer cells to escape and grow. Restoring the immune activity against cancer cells is the basis of Immunotherapy. How is this achieved?
To understand this, we need to understand the “Cancer Immunity Cycle” (as shown below)
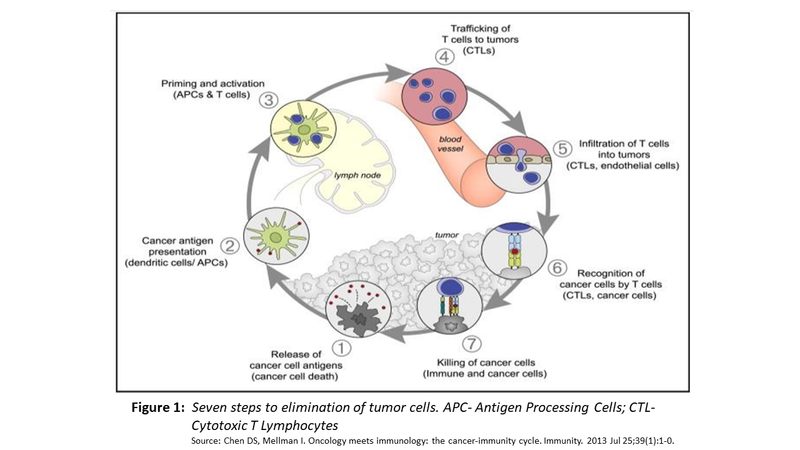
Step 1
- Tumour cells express MHC I/II restricted antigens on their surface. These antigens may be cancer-specific, caused by tumour specific genetic alterations, creating distinct peptides referred to as “Tumour Specific Antigens” (TSAs). Far more frequently, these antigens are normal or near normal peptides with low to no immunogenicity and are called “Tumour-Associated Antigens” (TAAs).
- In addition, tumour cells express antigens produced by stress response to DNA damage such as surface calreticulin and or NKG2D ligands recognised by CD8+ effector cells and NK cells, respectively. These interactions result in tumour cell lysis and abundant release of tumour antigens (as shown in Fig. 2)
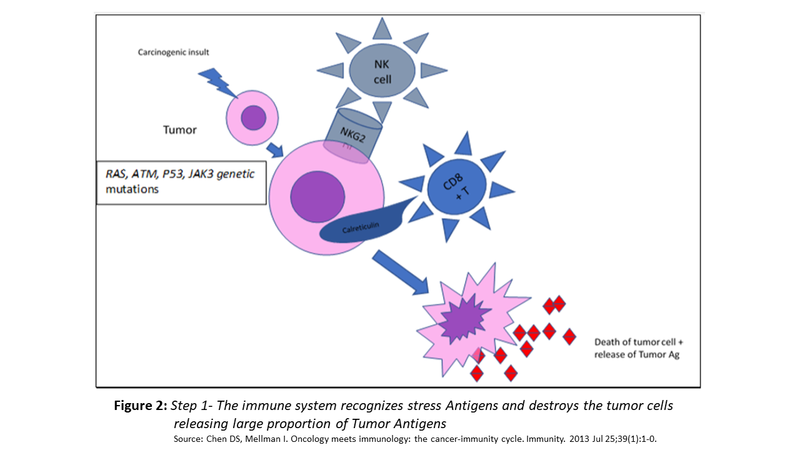
Step 2
- The released antigens are taken up by Antigen Presenting Cells (APC). The antigens are processed in the endoplasmic reticulum of APCs and expressed on their surface along with MHC class 1 molecules.
- Consequently, the dendritic cells are activated to secrete IL-12 and express CD1d on their surface.
- IL-12 invites NKT cells to tumour microenvironment and CD1d binds them to activate them to secrete IFN-ɤ. This cytokine inhibits the growth of the tumour and its angiogenesis.
- Far more significantly, the activated APCs leave the tumour microenvironment to reach regional lymph nodes and initiate the step 3.
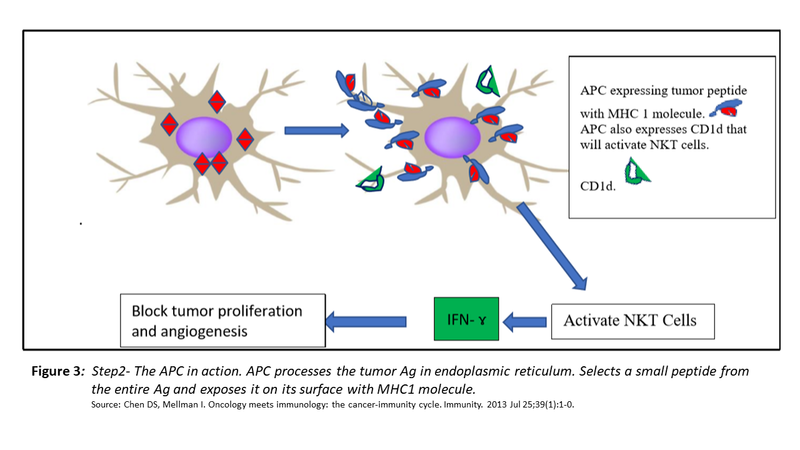
Step 3
- The activated APC leave the tumour via lymphatics to reach the regional lymph nodes.
- In the paracortical and interfollicular area of the lymph node, the APC interacts with CD3+, CD8+ cytotoxic T cells (CTL) by synapsing with T cell receptors (TCR) on latter with MHC class I restricted tumour antigen on their own surface. This signalling by itself though essential is not adequate to activate CTLs and requires costimulation by simultaneous binding of CD28 on CTLs with CD80/86 (B7) on APCs.
- This dual interaction educates the CTLs to specifically recognise the tumour antigens on any subsequent interactions and deliver the lethal kick to them. However, T cell unresponsiveness occurs in the absence of co-stimulatory signalling.

The crucial elements for efficient education of CTLs are:
- tumour antigens being sufficiently unique to be recognised as non-self
- the efficiency of APC to process them and express the most immunogenic peptide with class I MHC molecule
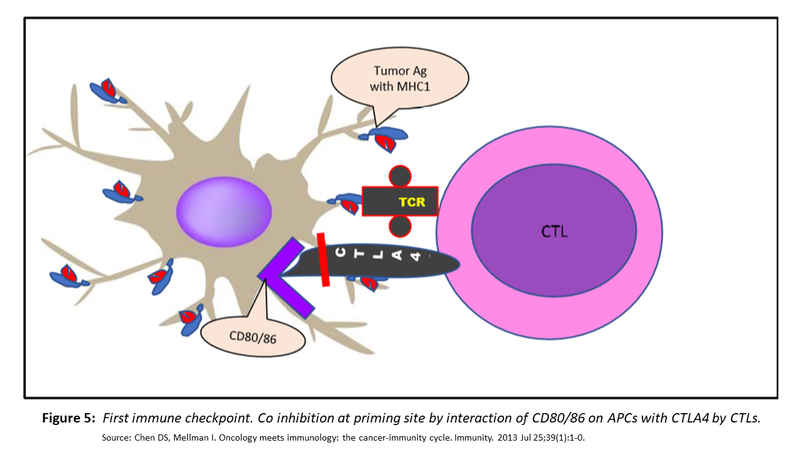
While, the effort to train the CTLs to perform their job effectively are underway, a balancing phenomenon so as to avoid an over-aggressive response is also initiated; a brake to ensure that activation of CTLs does not become an irrepressible process with autoimmune consequences. This inhibition of CTLs is brought about by the expression of CTLA4 by the CTLs that in turn binds with CD80/86 (B7).
Steps 4 & 5
The educated T cells in lymph node traffic out of the lymph node through high endothelial venules and find their way into circulation. Having reached the circulation, their final destination is the tumour microenvironment.
Steps 6 & 7
Once in the tumour microenvironment, the CTLS recognise the tumour cell antigens which they have been primed against by APCs and bring about the destruction of tumour cells by injecting perforin, granzyme and TIAs.
This leads to the additional release of antigens and deepening of the immune response. Even here, the effector function of CTLs is not allowed to extend interminably and is inhibited by synapsing of ‘Programmed Death Ligand 1’ (PDL1) expressed by tumour cells along with many cells in the tumour microenvironment with ‘PD1’ expressed on activated CTLs. The interaction is depicted in Fig. 6 (as shown below).
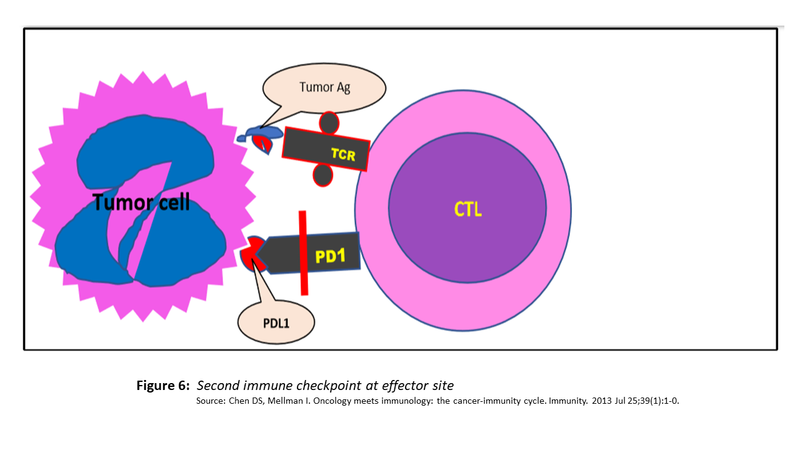
This checkpoint activation results in anergy, apoptosis of CTLs and their conversion to T regs cells which have immunosuppressive properties of their own.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
Dr. Anurag Mehta, is a senior pathologist with more than 30 years of experience in the field, and special interest in molecular biology and diagnostics is also the Director - Department of Laboratory & Transfusion Services and Director Research, Rajiv Gandhi Cancer Institute, N Delhi.
Dr. Garima Gupta is a Senior Research Officer at Rajiv Gandhi Cancer Institute, N Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries