Use of Immune Biomarkers in Cancer Treatment
M3 India Newsdesk Nov 13, 2023
The article stresses the crucial role of predictive biomarkers in guiding immunotherapy for advanced cancer, highlighting challenges and advocating for standardised techniques to enhance clinical benefits in specific patient groups.
Immunotherapy as a class of drugs has galvanised the field of oncology in the last few years. The impact is only matched to an extent by some of the monoclonal antibodies. Despite its wide-ranging benefits, only a limited number of patients are able to derive substantial benefits from these drugs. This could be due to various factors, some known and many unknown.
To optimise the selection of appropriate patients for immunotherapy and avoid unnecessary toxicity and healthcare costs, there is a clear need to identify truly predictive, and not simply prognostic, biomarkers of response.
Understanding which factors predict the clinical benefit of immunotherapy can improve the selection of tumour types and patient subsets who will respond, illuminate the mechanism of action of novel immunotherapeutic approaches, and potentially inform which patients require single-agent versus various combination strategies, which are increasing in availability.
Examples of biomarkers in the immunotherapy landscape include:
- Soluble factors such as serum proteins
- Tumour-specific factors such as receptor expression patterns and components of the microenvironment
- Host genomic factors
Many published analyses of potential predictive biomarkers for immunotherapy are retrospective, with limited extension into large prospective trials. In addition, there has been substantial variability in standardisation, measurement, and interpretation of early biomarker assays.
Furthermore, biomarker development in immunotherapy is challenged by the fact that immunotherapy targets are often inducible and dynamic over time and location. This is a function of the complex tumour microenvironment and the contribution of immuno-editing to the immune milieu.
Candidate biomarkers from serum, plasma, or peripheral blood must be accurately and reproducibly measurable, clinically feasible, cost-effective, and prospectively validated in randomised clinical trials.
Putative biomarkers in blood might be soluble factors such as serum proteins, circulating tumour DNA, or other cellular factors such as tumour cells, T-cell subsets, or other immune cell populations. The serum factors may be single or could include a panel of factors preferably measured by a single, validated assay.
Peripheral blood biomarkers
Serum soluble biomarkers
High pre-treatment levels of C-reactive protein (CRP), fibronectin and Vascular endothelial growth factor (VEGF) have been found to be inversely correlated with treatment outcomes with immunotherapy.
Peripheral blood cellular biomarkers
A high neutrophil to lymphocyte ratio, especially over 10 seems to forecast an even worse prognosis in patients undergoing immunotherapy, with sudden deaths in the weeks immediately following therapy.
Tumour cell phenotype and tumour microenvironment biomarkers
- Some tumours exhibit a T cell–inflamed phenotype. In these tumours, a large number of tumour-infiltrating lymphocytes (TILs) and chemokines that recruit T cells are found.
- Negative immune regulators including Fox P3+ regulatory T cells, PD-L1, and indoleamine-2,3-dioxygenase (IDO) are present as well; a type 1 interferon signature may also be present.
- In contrast, some tumours exhibit a non-T-cell–inflamed phenotype with an inverse pattern in which TILs are absent but chronic inflammation, as evidenced by common suppressive cytokines, tumour-associated macrophages, and myeloid-derived suppressor cells (MDSCs), exists.
- The T-cell inflamed phenotype is expected to respond well to immunotherapy.
Specific PD-1/PDL1 bioassays
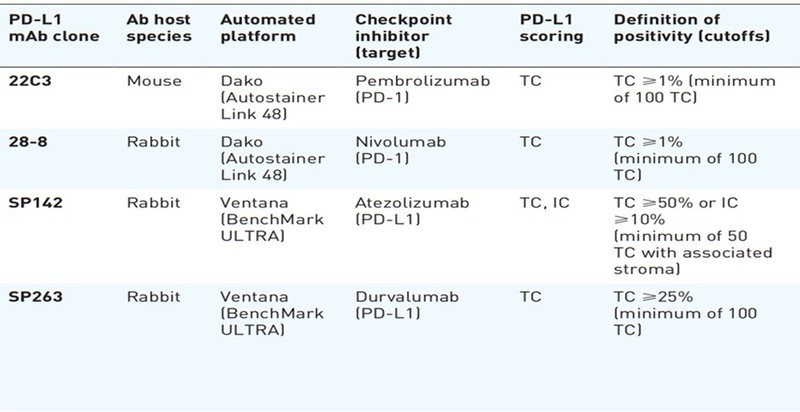
Various assays have been approved by the FDA for use as companion assays for specific immunotherapy drugs as shown in the table. Although it is recommended that the positivity and subsequent clinical decisions for a particular immunotherapy drug be based on recommended companion assays only, it is practically often unfeasible due to the unavailability of all assays in a particular lab.
Tumour genomic biomarkers
Microsatellite instability (MSI) results from a defective DNA mismatch repair (dMMR) system which leads to clusters of thousands of mutations along microsatellite regions.
Tumours with deficient MMR (dMMR) or high MSI (MSI-H) have increased mutational burden which leads to infiltration of T cells in the TME, leading to improved response with anti-PD1/PD-L1 therapies.
A higher tumour mutational burden (TMB) correlates with a greater probability of displaying neoantigens on the human leukocyte antigen (HLA) molecules of the tumour cell surface, eliciting CD8+ T cell-dependent immune responses and tumour cell lysis.
Importantly, TMB as a continuous variable does not have a linear correlation with OS, whereas PD-L1 expression has correlated with OS in advanced NSCLC patients.
Experimental tissue biomarkers
Immunoscore
“Immunoscore” was determined by quantification of cytotoxic and memory T cell populations within the tumour core and invasive margins and has been shown to be a prognostic marker in colorectal cancer independent of staging. Immunoscore is now being studied as a marker of response to immune checkpoint inhibitors (ICI) across tumour types.
Tumour Gene Expression Profiling (GEP)
GEP is a comprehensive approach to assess response to ICI using high-throughput tests to analyse immunologic transcriptomic patterns which predict sensitivity or resistance to ICI. Interferon-γ (IFN-γ) and related gene signatures have been assessed.
Microbiome
The gastrointestinal microbiota is important for anti-tumour immunity with many functions on both adaptive and innate immunity. Pre-clinical models have suggested the beneficiary effects of healthy microbiota in mouse models treated with immunotherapy.
Conclusion
Incorporation and standardisation of biomarker techniques, and multi-biomarker strategies to guide the selection of combination ICI approaches will facilitate the expansion of the clinical benefit of immunotherapy to appropriate subgroups of patients with advanced cancer.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Bipinesh Sansar, DM Medical Oncology, Associate Professor Medical Oncology at MPMMCC and HBCH, Varanasi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries