Unveiling the Unexpected: Decoding Hereditary Cancers
M3 India Newsdesk Jan 23, 2025
This article highlights the role of genetic mutations in hereditary cancers. It uses case discussions to illustrate diagnostic approaches and risk mitigation for affected individuals and their families.
Hereditary cancers account for 5-10% of all cancers and are caused by genetic mutations inherited from one generation to the next. These mutations often result in a predisposition to certain cancer types, offering a unique opportunity for early detection and prevention.
We have already discussed in our last article the chances of different types of cancers being hereditary in nature.
Cancer may be hereditary or genetic in nature if:
- Multiple family members are diagnosed with the same or related types of cancer.
- A germline mutation has been identified in the patient or their family. Even without a family history, hereditary cancer can be suspected if:
- Cancer develops at an unusually young age.
- Paired organs are affected.
- There are multiple primary cancers in a single individual.
- Features of a hereditary cancer predisposition syndrome are present. Specific cancer types include:
- Triple-negative breast cancer
- High-grade serous ovarian carcinoma
- Colorectal cancer with MSI-high
- Medullary thyroid carcinoma, retinoblastoma, neurofibromatosis, brain tumours, etc.
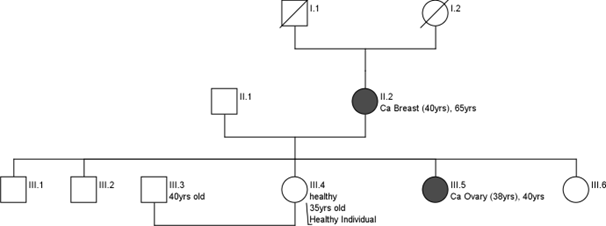
Case discussion 1
A 35-year-old healthy woman with a strong family history of breast cancer (Mother had Ca Breast at 40 years, sister had Ca ovary at 38 years).
- She came to the Cancer Genetics Clinic given her family history. A germline genetic test was recommended for which first a pre-test counselling was done for her.
- She has explained the implications of the test.
- Her personal history, family history and detailed pedigree were noted.
- Chances of being hereditary were explained.
- Basics of genetic tests, results expected and further management were also explained.
- She underwent a germline genetic test (next generation Sequencing).
Interpretation
A pathogenic heterozygous frameshift mutation in Exon 11 of the BRCA2 gene, Transcript NM_000059.3; c.5851_5854del p.(Ser1951Trpfs*11) was identified.
Recommendations
A Post-test counselling was done for her where she was explained the:
- Systemic Therapy
- Enhanced Screening
- Prophylactic surgeries once the family is complete
- Cascade family member testing
Case discussion 2
A 7-year-old child, with a family history of:
- His paternal uncle was diagnosed with osteosarcoma at age 15.
- His maternal grandmother was diagnosed with breast cancer at age 40.
- This child was brought to the clinic after his younger sibling was diagnosed with adrenocortical carcinoma (ACC).
Result
He and his family underwent genetic testing, which revealed a mutation in the TP53 gene, confirming a diagnosis of Li-Fraumeni Syndrome (LFS). This condition predisposes carriers to a variety of early-onset cancers, including sarcomas, breast cancer, brain tumours, and ACC.
Preventive and surveillance strategies
1. Regular cancer screening
Annual whole-body MRI scans to detect potential tumours early.
Regular ultrasound exams of the abdomen to monitor for adrenocortical tumours.
Brain MRIs every 6–12 months to check for early signs of brain tumours.
2. Lifestyle and environmental factors
Advised minimising exposure to ionising radiation (e.g., unnecessary X-rays or CT scans).
Encouraged a healthy diet rich in antioxidants to support cellular health.
3. Education and awareness
Taught the family to recognise early signs and symptoms of common cancers associated with LFS, such as persistent pain, lumps, or neurological symptoms.
4. Family counselling and psychosocial support
Discussed the implications of TP53 mutation for family planning and recommended preimplantation genetic testing (PGT) for future pregnancies to avoid passing on the mutation.
Case discussion 3
Patient history
A 42-year-old male presented with rectal bleeding, altered bowel habits, and unintended weight loss over three months. A colonoscopy revealed a tumour in the descending colon, and histopathology confirmed adenocarcinoma (stage III).
Family history
- Father: Diagnosed with colorectal cancer at 50 years.
- Maternal uncle: Died of gastric cancer at 54 years.
- Younger sister: Diagnosed with endometrial cancer at 38 years.
1. Initial testing with NGS
Comprehensive NGS panel targeting mismatch repair (MMR) genes (MLH1, MSH2, MSH6, PMS2, and EPCAM) and other CRC-related genes were performed.
Result: Negative for any pathogenic or likely pathogenic variants.
2. MLPA Analysis
Given the strong family history and clinical suspicion, MLPA was conducted to assess large deletions or duplications in the MMR genes.
Result: Positive for a heterozygous deletion in the MLH3 gene.
Discussion
This case underscores the limitations of NGS in detecting certain genetic alterations, such as large deletions, and highlights the role of MLPA in such scenarios. The identification of an MLH3 mutation not only guided the patient's management but also provided critical information for familial risk assessment and preventive strategies.
- A strong family history warrants further testing even if initial genetic tests are negative.
- MLPA is a valuable tool for detecting structural genetic alterations, especially in hereditary cancer syndromes.
- The identification of actionable mutations like MLH3 enables personalised management and risk reduction in both patients and their families.
This case exemplifies the need for a comprehensive and layered approach to genetic testing in hereditary cancer syndromes. The use of advanced diagnostics such as MLPA can bridge gaps left by NGS, ensuring accurate diagnosis and enabling tailored surveillance and prevention strategies for patients and their families.
Hereditary cancers present unique challenges but also opportunities for early detection and personalised prevention. Through genetic testing, counselling, and targeted management strategies, individuals and families can take proactive steps to mitigate their cancer risk. As research advances, the hope is to turn hereditary cancer predisposition into a manageable condition, improving outcomes for generations to come.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr. Archi Rungta, BDS, Currently pursuing a PG Diploma in Cancer Genetics and Genetic Counselling from Tata Memorial Hospital, Mumbai.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries