Understanding Pseudomembranous Colitis: A Case Study Presentation
M3 India Newsdesk Feb 08, 2024
The article provides a comprehensive overview of a case study involving a patient with pseudomembranous colitis due to Clostridioides difficile infection, focusing on the diagnosis, management, and treatment considerations for this challenging condition.
Case study
- A 53-year-old male, presented to the emergency department (ED) with worsening acute diarrhoea unresponsive to antibiotics.
- There was a repeated history of vomiting and diarrhoea in the past over-the-counter use of antibiotics for minor symptoms for the last 6 months and multiple times hospitalisation.
- His medical journey began two months ago at an external hospital, where he was admitted for acute appendicitis and a subsequent elective appendicectomy was scheduled. However, during the waiting period, the patient experienced a surge in bowel movements (four to five times daily). Despite an uncomplicated appendicectomy, diarrhoea intensified on postoperative day five.
- Testing revealed C. difficile via PCR, leading to a 10-day course of metronidazole and vancomycin. Completion of antibiotics did not alleviate symptoms, prompting further evaluation.
- Persistent issues included over 10 daily watery bowel movements with more than two tablespoons of blood, urgency, light brown to yellow-coloured stool, but no faecal incontinence, mucus, nausea, or vomiting.
- The patient denied travel, sick contacts, diet changes, or supplement use.
- A review of systems disclosed decreased appetite, unintentional weight loss (25 pounds in a month), and generalised fatigue.
- D/D: C. difficile infection, inflammatory bowel disease, ischemic colitis, postoperative complications, or malabsorption disorders.
- A collaborative approach involving gastroenterology and surgery is advised for a thorough assessment and personalised management plan.
- Upon admission, the patient presented with afebrility, tachycardia (105 beats per minute), hypotension (90/60 mmHg), and a saturation level of 98% on room air, indicating stable oxygenation.
- Although cachectic in appearance with bitemporal wasting and dry mucus membranes, the patient did not exhibit acute distress. Notably, an abdominal scar, showing no signs of drainage or erythema.
- Abdominal examination revealed a soft and non-tender abdomen with normoactive bowel sounds. There were no discernible skin lesions or lymphadenopathy.
- Digital rectal examination showed no external or internal lesions, erythema, normal sphincter tone, and the presence of liquid stool mixed with streaky blood on the digit upon removal.
- Laboratory findings were significant for hyponatremia (128 mEq/L), hypochloremia (96 mEq/L), indicative of malnutrition and dehydration (blood urea nitrogen 40 mg/dL, creatinine 1.6 mg/dL, and Albumin 2.5 g/dL).
- The patient also displayed normocytic anaemia (haemoglobin 10.6 g/dL, mean corpuscular volume 80.7 fL) and leukopenia (1.6 × 10^9/L). Elevated faecal calprotectin levels (800 μg/g) were noted, while C. difficile PCR toxin and antigen tests returned negative results.
- Other laboratory parameters, including human immunodeficiency virus, urinalysis, thyroid function, methicillin-resistant Staphylococcus aureus PCR, and procalcitonin, were within normal ranges.
- A contrast-free abdominal and pelvic computerised tomography (CT) scan revealed worsening pseudomembranous enterocolitis, corroborating the deteriorating gastrointestinal condition observed since the previous admission.
- At this juncture, the patient's clinical condition deteriorated further, marked by increased hypotension and leukocytosis (18.3 × 10^9/L).
- Consequently, he was promptly transferred to the Intensive Care Unit (ICU) with a preliminary diagnosis of fulminant C. difficile infection. In the ICU, the patient received treatment comprising the same regimen of oral vancomycin and intravenous metronidazole, augmented by vancomycin 500 mg enemas.
- Despite the severity of the situation, the patient did not necessitate the use of vasopressors, prompting a subsequent transfer back to the medical ward.
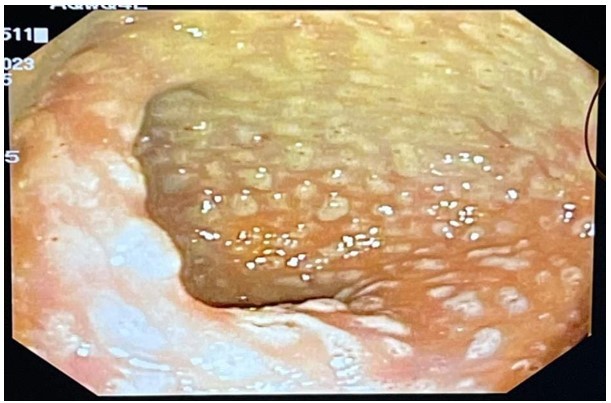
- A colonoscopy was performed, revealing diffuse yellowish, whitish plaques with fibrinous exudate. These endoscopic findings were consistent with the classic pseudomembranous colitis associated with C. difficile infection.
- Despite negative results from repeat testing for C. difficile, blood cultures, stool cultures, and ova and parasites, the pathology report provided crucial insights. It revealed a noticeable loss of cryptic architecture.
- Encouragingly, the patient exhibited improvement following a two-day course of fidaxomicin, leading to the decision to discontinue all other antibiotics. Subsequently, the patient underwent medical optimisation and was discharged with instructions to complete a full 10-day course of fidaxomicin.
Clostridioides difficile Infection (CDI): A growing challenge across generations
- Clostridioides difficile infection (CDI) has evolved into a prevalent nosocomial concern, contributing significantly to morbidity and mortality, particularly among older adults within hospital confines.
- However, increasing diagnosis not only in the elderly but also in younger patients and even within community settings.
- The colonisation of the human intestinal tract by C. difficile typically follows disruptions in the normal gut flora, often linked to antibiotic therapy. This insidious bacterium serves as the primary culprit behind antibiotic-associated colitis, encompassing severe manifestations such as pseudomembranous colitis.
Infection control guidelines for CDI:
- Healthcare professionals are advised to practice thorough hand hygiene, emphasising washing before and after patient contact.
- Notably, using soap and water is recommended over alcohol-based sanitisers due to C. difficile spores' resistance to alcohol.
Halting the precipitating antibiotic: A critical first move in CDI management
- Antibiotics, such as clindamycin, penicillins, fluoroquinolones, and cephalosporins, are typically associated with CDI, but the disease can occur with almost any anti-bacterial agent, including vancomycin and metronidazole, which are commonly used for treatment.
- A crucial initial measure in the treatment of Clostridioides difficile infection (CDI) involves the prompt discontinuation of the causative antibiotic agents.
- The concurrent use of antibiotics not targeted at CDI is linked to prolonged diarrhoea, heightened chances of treatment failure, and an increased risk of recurrent CDI.
Comprehensive care for CDI: Fluids, nutrition, and diarrhoea management
Supportive care: Prioritising correction of fluid losses and electrolyte imbalances is paramount.
Dietary considerations:
- Patients can follow a regular, low-residue diet as tolerated. This dietary approach aims to reduce stool frequency and volume.
- Notably, given that CDI primarily affects the colon, there is no mandatory requirement for instituting measures such as a lactose-free diet.
Antimotility agents:
- Traditionally, the use of antimotility agents like loperamide and diphenoxylate-atropine has been approached cautiously in CDI.
- These agents are reserved for cases where patients face challenges in maintaining fluid balance, particularly in the absence of ileus or colonic distention.
Assessment of disease severity:
Patients with acute CDI may exhibit systemic toxicity, with or without profuse diarrhoea, necessitating hospital admission, intensive care unit care, or even emergency surgery.
Nonfulminant disease:
Nonsevere CDI: White blood cell count ≤15,000 cells/mL and serum creatinine <1.5 mg/dL.
Severe CDI: White blood cell count >15,000 cells/mL and/or serum creatinine ≥1.5 mg/dL.
Fulminant colitis:
Definition: Previously referred to as severe, complicated CDI.
Indicators: Hypotension or shock, ileus, or megacolon.
Guidelines for initiating treatment for Clostridioides difficile Infection (CDI)
Indications for treatment:
Treatment is justified in patients displaying typical CDI manifestations, characterised by acute diarrhoea (≥3 loose stools in 24 hours) with no apparent alternative explanation and a positive diagnostic laboratory assay.
Considerations for empiric treatment:
- Empiric treatment is particularly prudent in situations where there is a strong clinical suspicion of CDI, even before diagnostic results are available.
- This is especially relevant for patients with symptoms indicative of severe or fulminant colitis.
- Timely intervention in such cases can be critical for favourable patient outcomes.
Caution against unnecessary treatment:
- It is crucial to note that treatment is not recommended for patients who test positive for CDI through diagnostic laboratory assays but do not exhibit diarrhoea or other CDI-related manifestations.
- Asymptomatic carriage is relatively common in such instances, and initiating treatment in the absence of symptoms may lead to unnecessary use of antimicrobial agents.
Hospital admission guidelines for CDI:
Fulminant CDI:
Immediate admission: All patients with fulminant CDI require prompt hospital admission for stabilisation, intravenous hydration, and antibiotic administration.
Mild CDI:
Outpatient management: Patients with mild CDI can often be managed in the outpatient setting, minimising the need for hospitalisation.
Consider hospitalisation for:
Severe CDI: Hospitalisation is advisable for patients with severe CDI symptoms requiring closer monitoring and intervention.
Frailty and dehydration: Admission should be considered for frail patients or those displaying signs of dehydration (e.g., low blood pressure, orthostasis, poor urinary output).
Limited social support: Patients lacking adequate social support, who may struggle to seek help if symptoms worsen, should also be considered for hospitalisation. This ensures timely medical assistance in case of deterioration.
The initial episode of nonsevere Clostridioides difficile Infection (CDI): Treatment approach
Regimen selection:
- The preferred options include oral fidaxomicin or oral vancomycin.
- Favouring fidaxomicin over vancomycin is recommended, aligning with the 2021 Infectious Diseases Society of America (IDSA) guidelines.
- This preference is driven by a slight advantage in recurrence rates associated with fidaxomicin.
Considerations for high-risk subgroups: Patients at a higher risk for recurrence, such as those aged over 65 years, individuals with compromised immunity, severe CDI cases, or those with ribotype 027/078/244 infections, may derive increased benefits from fidaxomicin.
Alternative agent:
- Metronidazole serves as an alternative but less effective agent for treating nonsevere CDI when oral vancomycin and oral fidaxomicin are not available.
- However, its use is cautioned in patients who are frail, aged over 65 years, or those who develop CDI in association with inflammatory bowel disease.
Ineffectiveness of intravenous vancomycin:
Intravenous vancomycin is not recommended for the treatment of CDI as the drug is not excreted appreciably into the colon during short-term systemic administration, rendering it ineffective in this context.
Duration and follow-up in nonsevere Clostridioides difficile Infection (CDI) treatment
Duration of antibiotic therapy:
- For nonsevere CDI, the recommended duration of initial antibiotic therapy is 10 days.
- However, patients with CDI occurring concurrently with another underlying infection necessitating prolonged antibiotic therapy are at an elevated risk for recurrent CDI.
- In such instances, the standard practice involves continuing CDI treatment throughout the antibiotic course, with an additional tail of one week post-completion.
Follow-up protocol:
- In patients showing signs of recovery or whose symptoms have resolved, repeating stool assays during or after treatment is unnecessary.
- It is important to note that up to 50 per cent of patients may exhibit positive stool assays for as long as six weeks following the completion of therapy.
- Therefore, a lack of symptoms should guide the decision on the need for further testing.
- Monitoring for symptom resolution and clinical improvement serves as a more reliable indicator than routine stool assays in these cases.
Management of severe Clostridioides difficile Infection (CDI)
Antibiotic therapy:
Regimen selection:
For patients facing an initial episode of severe CDI, oral fidaxomicin is suggested over oral vancomycin, aligning with the 2021 Infectious Diseases Society of America (IDSA) guidelines.
Dosing regimens:
- Both fidaxomicin and vancomycin can be administered using standard dosing or in an extended-pulsed regimen.
- The use of tigecycline for C. difficile colitis treatment is not favoured due to insufficient data.
Monitoring during treatment:
- In cases of severe or fulminant colitis where mucosal disruption is present, systemic absorption of enteral vancomycin is possible.
- Monitoring serum vancomycin levels is favoured for patients with renal failure, severe colitis, and a prolonged course (>10 days) of enteral vancomycin therapy.
Duration of antibiotic therapy:
- The standard duration of antibiotic therapy for an initial CDI episode is 10 days, whether using vancomycin or fidaxomicin.
- However, the duration of the antibiotic course should be individualised for patients with severe disease based on their response to therapy and clinical course.
For those with underlying infections requiring prolonged antibiotic administration, CDI treatment should continue throughout the antibiotic course plus one additional week after completion.
Role of Fecal Microbiota Transplantation (FMT) in severe and fulminant CDI
FMT has emerged as an alternative to colectomy in patients with severe and fulminant colitis, demonstrating potential reductions in mortality, as suggested by retrospective and observational studies.
Administration considerations:
- FMT instillations are ideally administered via colonoscopy due to the larger volume of stool that can be delivered into the colon compared to oral capsule administration.
- Additionally, potential issues such as atonic colon or ileus may impede orally administered faecal material from reaching the colon.
- Multiple stool infusions may enhance efficacy compared to a single infusion.
Surgical considerations in Clostridioides difficile Infection (CDI)
Indications for early surgical consultation:
- Hypotension
- Fever ≥38.5°C
- Ileus or significant abdominal distention
- Peritonitis or significant abdominal tenderness
- Altered mental status
- White blood cell count ≥20,000 cells/mL
- Serum lactate levels >2.2 mmol/L
Admission to the intensive care unit
- End organ failure (e.g., requiring mechanical ventilation, renal failure)
- Failure to improve after three to five days of maximal medical therapy
Bezlotoxumab in Clostridioides difficile Infection (CDI) management
Bezlotoxumab, a humanised monoclonal antibody targeting C. difficile toxin B, gained approval from the US Food and Drug Administration in 2016. Its role in CDI management involves a one-time infusion alongside a standard treatment regimen.
Administration and caution:
- Bezlotoxumab is administered as a single infusion, complementing the standard treatment approach.
- However, caution is advised when considering its use in patients with congestive heart failure.
- The infusion of bezlotoxumab aims to mitigate the recurrence of CDI, particularly in high-risk populations.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Jimmy Patel is a practising gastroenterologist in Chennai.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries