Steroids over Tocilizumab : IDSA's latest recommendation for COVID-19
M3 India Newsdesk Sep 30, 2020
The Infectious Disease Society of America has updated the recommendation for glucocorticoid for the management of COVID-19. Dr. Monish Raut provides a explains the recent update basis the two trials that influenced the change in recommendation.
For our comprehensive coverage and latest updates on COVID-19 click here.
Earlier experience of SARS and MERS and then initial days of SARS-CoV-2 warned against the use of systemic corticosteroids due to the possibility of deteriorating clinical condition. The major clinical, pathological effects of COVID-19 is particularly due to hyperinflammatory response also called as cytokine storm. Immunomodulatory therapy using corticosteroids have been found to be useful in recent trials to reduce mortality.
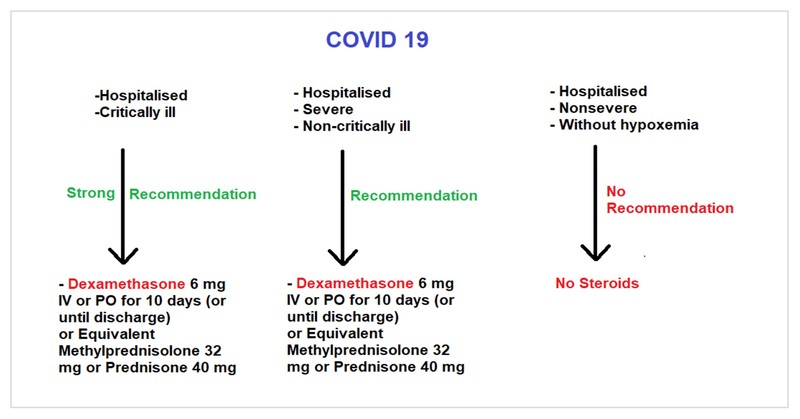
- Critical illness: Patients on mechanical ventilation and ECMO, having sepsis and ARDS
- Severe illness: Patients having saturation ≤94% on room air and needing supplemental oxygen
- Non-severe illness: Patients having saturation >94% not needing supplemental oxygen
Basis of the recommendation
Various randomised control trials including the RECOVERY trial have found that glucocorticoid could reduce mortality at 28 days by 34% and 17% in critically ill and severely ill COVID-19 patients. However, no benefits were noticed in non-severely ill patients without hypoxia.
It is also worth mentioning that hyperglycaemia, neurological side effects (e.g. agitation/confusion), the risk of bacterial and fungal infection and adrenal suppression are experienced by patients.
Role of tocilizumab in COVID -19
COVID-19 patients with raised IL6 levels were found to have more severe disease. Tocilizumab is a monoclonal anti-IL-6-receptor blocking antibody which can ameliorate the hyperinflammatory response. There have been studies concerning tocilizumab of contradictory proof of gain in patients of COVID-19.
IDSA guideline advises against the routine use of tocilizumab in hospitalised COVID-19 patients.
Basis of recommendation
One randomised, controlled trial failed to show the benefit on mortality of hospitalised COVID-19 patients by using tocilizumab. Adverse effects with tocilizumab were elevated; risk of fungal, viral, bacterial, and mycobacterial infections, some reports of gut perforation in non-COVID patients have also been mentioned.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Monish S Raut is a Consultant in Cardiothoracic Vascular Anaesthesiology. His area of expertise is perioperative management and echocardiography with numerous publications in various national and international indexed journals.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries