Round-up of COVID-19 vaccines - Comparing the 6 top contenders: Dr. Purvish Parikh
M3 India Newsdesk Dec 08, 2020
Dr. Purvish Parikh compares data available from studies conducted by 6 potential vaccine candidates- moving faster than ever before, to deliver an effective innoculate that will eventually end the fight against the COVID pandemic.
To read other originals by Dr. Purvish Parikh, click here.
For our comprehensive coverage and latest updates on COVID-19 click here.
While we still struggle to understand how soon COVID-19 vaccine shall be available to us, airlines are already gearing up to capitalise on the opportunity to transport them worldwide. At the same time, social media is buzzing with the controversial statement that the COVID-19 pandemic shall be over before the vaccines become available.
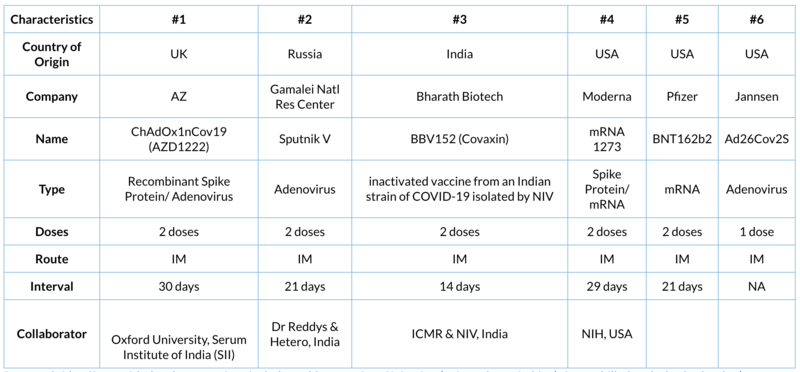
Notwithstanding the two sides of the argument, this article shall focus on the current status of the various vaccines that might see the light of the day in the near future. So, in case you need to use the vaccine (for yourself, your family or your patients) you shall understand the unique characteristics of each of them. Today, there are at least 187 vaccine candidates (according to WHO) being studied of which 44 are in advanced phases of human clinical trials. We will focus on six vaccines (excluding the three developed by china).
 Reported side effects with the above vaccines include problems at site of injection (pain, redness, itching), fever, chills, headache, bodyache (syn muscle pain, soreness), nausea, and vomiting. These are common side effects with a majority of vaccines that we have been using since several decades. They can occur in upto 50% of people but are usually mild and subside on their own in a short while. Rarely does it cause symptoms that require medication.
Reported side effects with the above vaccines include problems at site of injection (pain, redness, itching), fever, chills, headache, bodyache (syn muscle pain, soreness), nausea, and vomiting. These are common side effects with a majority of vaccines that we have been using since several decades. They can occur in upto 50% of people but are usually mild and subside on their own in a short while. Rarely does it cause symptoms that require medication.
- The UK AstraZeneca in collaboration with Oxford University vaccine trial was sailing smoothly with a 90% efficacy claim. It had planned to study 30,000 individuals. The trial was temporarily put on hold when one of the participants developed transverse myelitis. Indian Council of Medical Research (ICMR) inquired into this alleged adverse event, but did not find it related and hence recommended continuation of the trial. Unfortunately, that was not the only problem with this vaccine. Now, a very significant "error" has been identified that puts into question the data integrity as well as the quality oversight.
- The first is that the actual efficacy in one segment of the trial could actually be much lower - at 62%
- The other controversy is the suggested claim that the first dose required is only half the recommended dose
In the meantime, this vaccines Indian partner, SII is planning to apply for emergency use license to DCGI in a few weeks.
- Sputnik V claims to be the first COVID-19 vaccine that has been granted approval by the regulatory authorities (11th August 2020). Its trials includes patients from Russia, India, Hungary, UAE, Armenia, Venezuela and Belarus. Its efficacy was recently endorsed by British scientists. Russia has agreed with Hetero to allow it to manufacture at least 100 million doses in India. The other Indian partner, Dr. Reddy's says that the final phase trial results will be ready by March 2021. This vaccine is expected to be internationally priced significantly less than the other vaccines being developed by the western world, a significant benefit for millions of persons and their countries.
- Bharat Biotech's COVID-19 vaccine is in phase 3 at 25 centres across India and shall study 28,500 subjects. It is an inactivated vaccine which has been developed from one of the Indian strains of COVID-19 that was isolated by NIV, Pune. This is the first vaccine candidate from India to enter phase 3 clinical trial development state. AIIMS, New Delhi was the first centre to commence the phase 3 trial amongst volunteers from its faculty. If this is as successful as promised, it would be a proud moment for Make in India.
- Moderna vaccine was the first to be tested in humans in the USA. The company was recently established, has no prior track record in vaccine production, is using a novel (and largely untested mRNA method of production) and is essentially funded by NIH, USA. It also does not have any medicinal product that is approved by US-FDA nor have their facilities ever been inspected by the regulatory agency. They also intend to study the vaccine in 30,000 individuals.
- Pfizer's vaccine is also planned to be studied in 30,000 individuals across 120 centres. Data is mature enough for the company to file for regulatory marketing approval in the USA under emergency use provision. The US-FDA advisory board is to meet on December 10th to take a decision. They have also submitted "amended protocol" to US-FDA to increase the number of patients in their phase 3 trial to 44,000 persons. The biggest hurdle for this vaccine is the need to store and ship the product at -70 degree centigrade.
- Janssen's vaccine candidate is being evaluated in 60,000 subjects from Brazil, Chile, Columbia, USA, Mexico, Philippines, Peru, and South Africa. The biggest advantage of this vaccine could be that it is required to be administered only once (single dose). It was halted after a patient developed a serious adverse event. After thorough evaluation by the regulatory authorities, it was decided that the relationship of the SAE to the vaccine could not be established. Hence the phase 3 trial was given permission in October to restart.
In conclusion, the light at the end of the 'COVID-19 pandemic tunnel' seems to be brightening. The next six months shall decide the fate of many across the globe. We must remind ourselves that vaccine studies can look at primary end points of reducing infection, disease and/or transmission.
The final proof of the pudding shall be efficacy in reducing or preventing severe disease or death - a hard endpoint that is unlikely to be assessable in phase 3 clinical trials. In the meantime, we live with the hope to continue to take optimal precautions and look forward to being vaccinated safely and quickly.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Purvish Parikh is a Precision & Medical Oncologist from Mumbai.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries