Influenza Vs. Mutations
M3 India Newsdesk Jan 25, 2024
Explore the dynamic interplay of influenza virus evolution in this insightful article. From antigenic drift to the dance of type A vs. type B viruses, gain key insights into the challenges, implications for public health, and the ongoing battle against the ever-evolving flu landscape.
In the ever-evolving realm of viruses, the influenza (flu) virus distinguishes itself with a remarkable ability to undergo mutations, giving rise to new strains that challenge our immune defences. Understanding the dynamics of influenza virus mutations is paramount in our ongoing battle against seasonal outbreaks and occasional global pandemics.
Antigenic drift: A stealthy evolutionary twist in influenza viruses
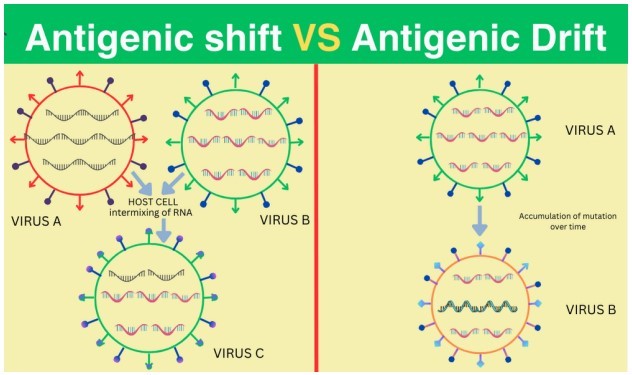
Influenza viruses exhibit remarkable adaptability, one key attribute being their propensity for antigenic drift. This phenomenon involves the accumulation of minor genetic mutations in viral genes, predominantly those encoding surface proteins hemagglutinin (HA) and neuraminidase (NA). These "antigenic" proteins engage with the immune system, triggering the production of antibodies. Over time, the gradual piling up of these mutations leads to the emergence of new, slightly divergent viral strains. Influenza vaccines are meticulously designed to target specific antigenic configurations displayed on circulating viral strains.
Challenges posed by antigenic drift
The subtle modifications wrought by antigenic drift can render these "keyholes" unrecognisable from the pre-programmed "immune locks." This explains why individuals may experience multiple influenza episodes throughout their lives, as each drifted strain represents a novel antigenic challenge.
Adaptations in influenza vaccine composition
To maintain efficacy in this ever-shifting landscape, influenza vaccine composition undergoes annual revisions, meticulously tracking the evolutionary dance of the virus.[1]
Antigenic shift: A major viral makeover
Influenza viruses possess a remarkable capacity for evolution, not just through gradual antigenic drift but also through more dramatic transformations known as antigenic shifts. These shifts involve the emergence of entirely new hemagglutinin (HA) and/or neuraminidase (NA) protein configurations in viruses that can infect humans. Unlike drift, which entails modest genetic changes within the same subtype, a shift can introduce a distinctive subtype of flu A virus, one unfamiliar to the human immune system.
Origins of antigenic shifts
Antigenic shifts typically occur when a flu virus from an animal reservoir, such as birds or pigs, acquires the ability to jump species and infect humans. These zoonotic viruses may possess HA or HA/NA combinations so divergent from circulating human influenza viruses that most individuals lack pre-existing immunity.
Illustrative example
A prime example of this phenomenon unfolded in 2009, when an H1N1 virus, harbouring genetic material from swine populations across continents, emerged and triggered a global pandemic, demonstrating the unsettling potential of antigenic shifts for widespread outbreaks.[1]
The dance of evolution: Type A vs. type B
Influenza virus classification
Influenza viruses are classified into types A, B, and C.
- Type A viruses: Capable of causing pandemics due to antigenic drift and shift, evolve more dynamically than type B viruses.
- Type B viruses: Change more gradually, primarily through antigenic drift, and are not associated with pandemics.
Challenges in public health
The constant genetic evolution of flu viruses, driven by antigenic drift and shift, poses a significant challenge for public health efforts. The delicate dance between the virus and our immune system underscores the need for ongoing surveillance, research, and adaptive strategies to stay one step ahead of these ever-changing pathogens.[1]
Navigating the flu landscape: Implications for public health
Understanding influenza virus mutations
As we delve into the intricate realm of influenza virus mutations, the implications for public health become evident. The dynamic nature of these mutations poses challenges in crafting effective prevention and control measures, demanding a nuanced and adaptive approach.
Critical role of annual flu vaccinations
Annual flu vaccinations, tailored to anticipate and counteract evolving strains, serve as a critical line of defence against seasonal outbreaks. Public health agencies worldwide engage in continuous surveillance, monitoring genetic changes in circulating flu viruses to ensure vaccines remain effective.
Advancements in genomic sequencing
Advancements in genomic sequencing technologies play a pivotal role in enhancing our understanding of influenza virus mutations. Real-time monitoring allows researchers to identify emerging strains and assess their potential impact on public health.
Proactive approach and targeted interventions
This proactive approach enables the development of targeted interventions, such as timely updates of vaccine formulations, to align with the evolving viral landscape.[1,2,3,4]
Treatment and challenges: Navigating the evolving landscape
While influenza viruses lack a traditional cure, vaccinations remain a critical element in the battle against the flu. The constant evolution driven by antigenic drift and shift poses challenges for vaccine development and effectiveness.
Role of annual flu vaccinations
Annual flu vaccinations play a pivotal role in preventing and mitigating the impact of seasonal outbreaks. However, the dynamic nature of antigenic shifts, leading to the emergence of entirely new virus subtypes, presents a formidable challenge.
Addressing challenges
Challenges associated with antigenic shifts highlight the importance of swift response mechanisms, international collaboration, and the development of rapid vaccine formulations to address novel strains.
Emerging trends in vaccine development
Emerging trends in vaccine development, including mRNA vaccine platforms, hold promise in providing a more adaptable and rapid response to evolving influenza viruses.[1,4]
Conclusion: A constant vigilance
- In conclusion, the constant evolution of the influenza virus through antigenic drift and shift underscores the need for constant vigilance and adaptability in our approach to public health.
- The dance between the virus and our immune system is a dynamic interplay, and our ability to navigate this landscape relies on scientific advancements, global collaboration, and a commitment to staying one step ahead of these elusive and mutating pathogens.
- As we unravel the mysteries of influenza virus mutations, our collective efforts in research, surveillance, and vaccination serve as crucial pillars in the ongoing fight against the flu.
- By remaining vigilant and responsive, we can mitigate the impact of seasonal outbreaks and enhance our preparedness for potential future pandemics.
- The ever-changing nature of influenza viruses demands a proactive and informed stance, ensuring that our strategies for prevention and control remain effective in the face of this perpetual evolutionary dance. [1,2,3,4]
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Sarthak Chakravarty is a practising general physician with a specialisation in Emergency medicine & Trauma.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries