Infertility: Stepwise Evaluation and Case Studies
M3 India Newsdesk Mar 13, 2023
Although the exact cause of infertility may be difficult to pinpoint, it may be due to problems with ovulation in women and/or low levels of a few hormones in both men and women. This article provides a detailed evaluation of factors responsible for infertility with the help of a case study.
Up to 15% of couples have infertility, which is defined as failure to conceive within 12 months of unprotected sexual activity or therapeutic donor insemination in women under 35, or within 6 months in women over 35.
Women older than 35 years should receive an expedited evaluation and undergo treatment after 6 months of failed attempts to become pregnant or earlier if clinically indicated.
In women older than 40 years, more immediate evaluation and treatment are warranted. Additionally, if a woman has a condition known to cause infertility, the obstetrician–gynaecologist should offer immediate evaluation. Indications for immediate evaluation include the following:
- Oligomenorrhea or amenorrhea
- Known or suspected uterine, tubal, or peritoneal diseases
- Stage III or stage IV endometriosis
- Known or suspected male infertility
Factors responsible for infertility
1. Male factors – 30%
2. Female factors – 30%
3. Combined (both male and female responsible) – 30%
4. Unexplained – 10%
Basic infertility evaluation
Female:
- History
- Physical Examination
- Prepregnancy evaluation
- Evaluation for aetiology of infertility
- Diminished ovarian reserve
- Ovulatory dysfunction
- Tubal factors
- Uterine factors
Male:
- History
- Examination
- Semen analysis
History
- Duration of infertility and results of any previous evaluation and treatment.
- Menstrual history (including age at menarche, cycle interval, length, and characteristics; the presence of mild premenstrual symptoms; and onset and severity of dysmenorrhea), signs of ovulation including positive ovulation tests, cervical mucus changes, or biphasic basal body temperatures.
- Pregnancy history (gravidity, parity, time to pregnancy, fertility treatments, pregnancy outcome, delivery route, and associated complications).
- Previous methods of contraception.
- Coital frequency and timing.
- Sexual dysfunction.
- Past surgery (procedure, indication and outcome) especially abdominal and pelvic procedures.
- Gynecologic history (eg, pelvic inflammatory disease, sexually transmitted infections, endometriosis, leiomyomas).
- Sexual history.
- Previous abnormal cervical cancer screening tests and any subsequent treatment.
- Current medications and supplements, with an emphasis on identifying allergies and potential teratogens.
- Family history of birth defects, developmental delay, early menopause, or reproductive problems.
- Occupation and exposure to known environmental hazards and use of nicotine products, alcohol, and recreational or illicit drugs.
Examination
- Weight, body mass index, blood pressure, and pulse
- Thyroid enlargement and the presence of any nodules or tenderness
- Breast secretions and their character
- Signs of androgen excess
- Tanner's staging of breasts and pubic and axillary hair
- Vaginal or cervical abnormality, secretions, or discharge
- Pelvic or abdominal tenderness, organ enlargement, or masses
- Uterine size, shape, position, and mobility
- Adnexal masses or tenderness
- Cul-de-sac masses, tenderness, or nodularity
Causes of female factor infertility
1. Ovulatory dysfunctions
Accounts for 30- 40% of all cases of female infertility. Tests for ovulation include:
- History
Regular menstruation occurring at 25 – 35 days intervals along with mild to moderate dysmenorrhoea and premenstrual symptoms are good predictors of regular ovulation. Mid-cycle pelvic pain (Mittelschmerz syndrome) is associated with Ovulation.
- Basal Body Temperature (BBT)
The BBT falls by 0.25̊C or 0.5̊F during ovulation and then rises 0.25̊C or 0.5̊C (0.5̊F to 1̊F) after ovulation. This biphasic temperature is predictive of ovulation and is due to the thermogenic progesterone hormone in the luteal phase.
- Cervical mucous study
Cervical mucous has great elasticity and can be stretched up to 10cm at the time of ovulation due to estrogenic activity. In the luteal phase, the mucous becomes viscous, and tenacious and loses its elasticity. So, the disappearance of Spinnberkeit is evidence of ovulation.
- Hormonal studies
- Serum progesterone levels on D21 of a 28days cycle show the peak of mid-luteal serum progesterone. A level of >10 ng/ml is indicative of ovulation and normal corpus luteal activity. Level <5ng/ml indicates corpus luteal deficiency.
- Urinary LH kits are used to detect mid-cycle LH surges in urine. LH surge occurs 24 hours before ovulation
- Endometrial biopsy
It is done 5-7 days before menstruation (day 21 in a 28-day cycle or day 28 in a 35-day cycle) or during the first 6 hours of menstruation. The sample is divided into two parts. One part is sent in normal saline for AFB staining and PCR for Mycobacterium tuberculosis. Another part is sent in 10% formalin for histopathology. Proliferative endometrium and sub-nuclear vacuolation are diagnostic of ovulation.
- Diminished ovarian reserve
The presence of decreased ovarian reserve predicts future response to ovarian stimulation. The results of ovarian reserve tests should be considered in the context of the patient’s age. Although there are no definitive criteria for diminished ovarian reserve, the following values may be considered consistent with diminished ovarian reserve:
- Antimüllerian hormone (AMH) value less than 1 ng/mL
- Antral follicle count less than 5–7 and
- Follicle-stimulating hormone (FSH) greater than 10 IU/L or
- A history of poor response to in vitro fertilisation stimulation (fewer than four oocytes at the time of egg retrieval)
2. Tubal patency test
- Hysterosalpingography (HSG)
It is time tested, inexpensive, simple, and primary test for uterine and tubal evaluation. Radiopaque contrast is injected into the cervix under fluoroscopic guidance. Proximal and distal tubal occlusion, peritubular adhesions, and salpingitis isthmic nodosa may be seen with HSG. The positive predictive value and negative predictive value of HSG for assessing tubal patency have been estimated as 38% and 94%, respectively.
- Saline Infusion Sonography (SIS)
It is performed in the follicular phase of the cycle under ultrasound control. 200ml of normal saline is infused into the uterine cavity with the help of a Foley catheter. Under ultrasound guidance, it is possible to visualise the Fallopian tubes by following the flow of saline in the uterus and out of the tubes which suggest tubal patency. It is particularly useful in diagnosing submucous/ pedunculated myoma or polyp.
- Hystero-Salpingo-Contrast Sonography (HYCOSY)
In this technique an ultrasound contrast medium (like Echovist containing galactose microparticles) is infused into the uterine cavity with a Foley catheter or a cannula and the flow of medium into the uterine cavity, fallopian tubes and the peritoneal cavity is observed by ultrasound.
- Laparoscopy and chromotubation (Dye testing)
It is popularly called Lap and Dye test. Laparoscopy is the gold standard method for accurate assessment of the condition of fallopian tubes, uterus, ovaries and other pelvic structures. Chromotubation is done at the same sitting using an HSG cannula or Foley catheter and using methylene blue dye.
- Falloposcopy
Falloposcopy is defined as transvaginal microendoscopy of the fallopian tubes and direct visualisation of the entire fallopian tube lumen. It is always done under laparoscopic guidance.
- Salpingoscopy
Salpingoscopy is the passage of a very fine rigid endoscope through the fimbrial end of the tube. It is done under laparoscopic guidance and helps to visualise the lining epithelium up to the asthma-ampullary region.
3. Tests for uterine factors
Although these are relatively uncommon reasons for infertility in women, abnormalities of the uterine anatomy or function should be ruled out:
- Transvaginal sonography (TVS)
- HSG
- Saline infusion sonography
- Hysteroscopy- definitive method for diagnosis and treatment of intrauterine pathology. As it is a costly and invasive method, it is reserved for further evaluation and treatment of abnormalities defined by HSG and SIS
- 3D USG and MRI
4. Combined laparoscopy and hysteroscopy
It has become a gold standard to evaluate the uterine, tubal and pelvic factors of infertility as well as to perform the surgery if needed (septal resection, adhesiolysis and ablation of endometriosis).
5. Cervical factors
Abnormalities of cervical mucous production or sperm mucous interaction rarely are the sole cause of infertility.
Postcoital Test (Sims Huhner test)- Cervical mucous is drawn by pipette within 12 hours of coitus and examined under a high-power microscope. The presence of ≥10 progressive motile sperms/hpf is normal and called positive, no sperm or all dead is abnormal and called negative, immotile sperm with normal sperm count indicates the presence of anti-sperm antibody. It is rarely performed nowadays because of poor predictive value, lack of reproducibility, poor sensitivity, lack of uniform methodology and strict criteria of assessment. Most importantly this test does not change the management protocol.
The male factor of infertility
Causes of male infertility
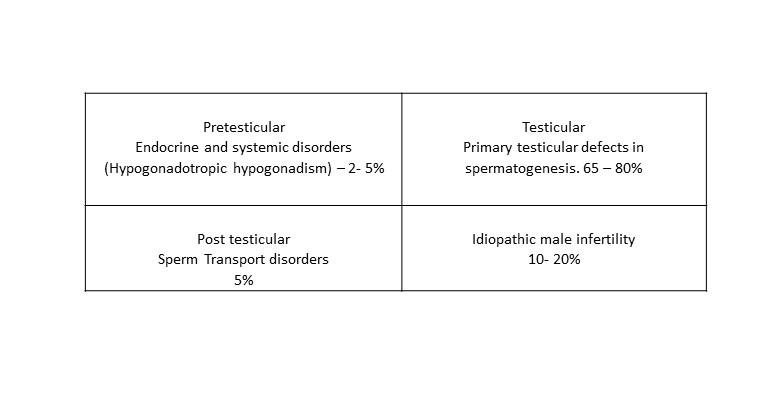 History
History
The following list includes particular examples of important historical male variables to elicit;
- Coital frequency and timing
- Any evidence of sexual dysfunction, including erectile or ejaculation issues
- Duration of infertility
- Prior fertility
- Childhood illness and developmental history
- Systemic medical illness
- Previous surgery (eg, cryptorchidism with or without surgery) medication use, including anabolic steroids and supplements (eg, testosterone) and allergies
- Sexual history and sexually transmitted infections and
- Exposure to gonadal trauma or toxins
Examination
- Height, weight, obesity
- Secondary sexual character. Gynaecomastia
- The thyroid gland, galactorrhoea, visual defect and features of endocrinopathy
- Hernia, lymph node in the groin region
- Scrotum- hernia, hydrocele and varicocele.
- Testes: Size and sensation
- Penis: hypospadias, phimosis
- Epididymis and vas
- A rectal examination is done to palpate the prostate and seminal vesicles
The World Health Organisation’s accepted reference value for semen analysis, 2010
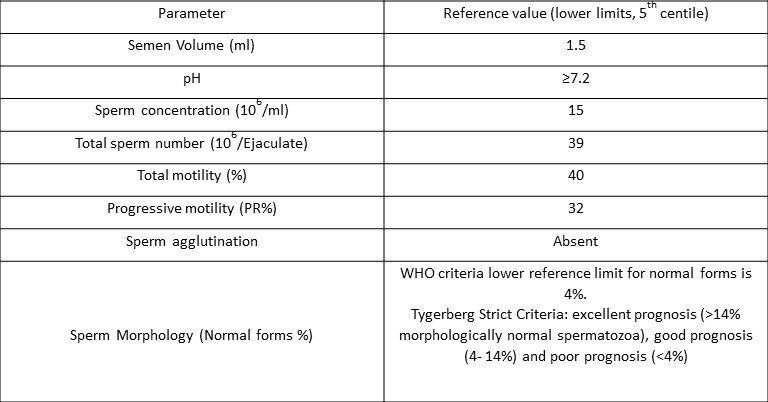
The semen sample should be collected after 2 to 5 days of abstinence and should be submitted to the laboratory within one hour of collection.
Unexplained infertility
Up to 30% of infertile couples may have an undiagnosed infertility diagnosis. When the criteria for infertility are met, the fundamental infertility assessment is carried out, and all the test findings are normal, the condition is known as unexplained infertility. At a minimum, these patients should have evidence of ovulation, tubal patency, and a normal semen analysis 5.
Case scenario
A patient, 28yrs old, married for 3 years, attended ESIC-PGIMSR GOPD in 2018 with a complaint of stoppage of menstruation for 3 years and the inability to conceive for 2 years. At the age of 20, the patient was diagnosed as Hypothyroid and was put on T. Eltroxine 100mcg. Secondary sexual characteristics normal, BMI 28.6Kg/m2 with no signs of Hyperandrogenism like Acne, Hirsuitism, Alopecia (PCOS)
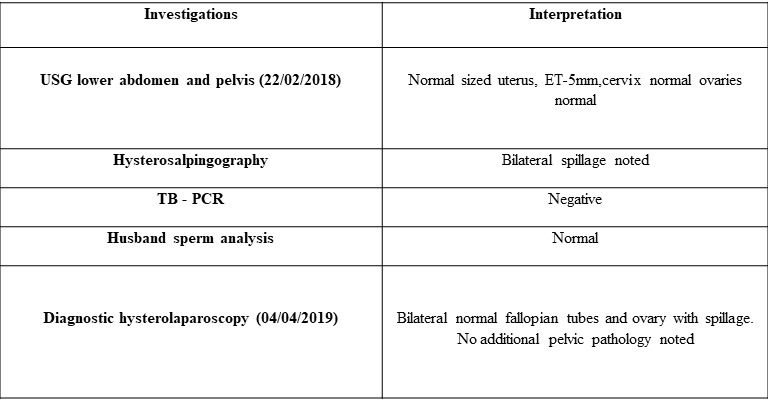
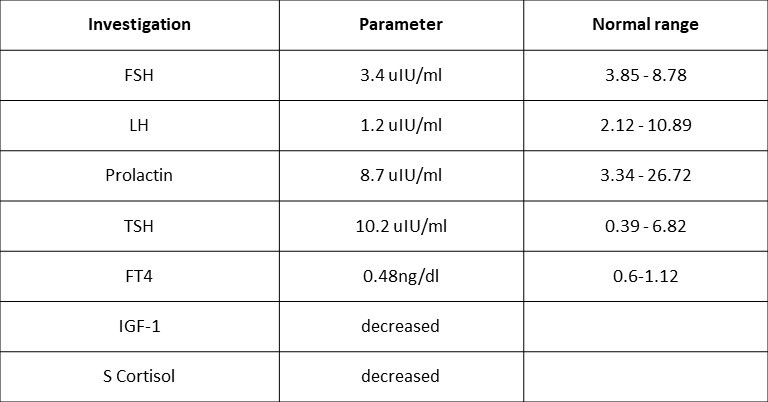
- Investigations were suggestive of hypogonadotropic hypogonadism, the patient was advised MRI.
- MRI on 30/08/2018 suggested empty sella with extremely hypoplastic anterior lobe of pituitary.
- The patient was started on T. Prednisolone 5mg OD to prevent further progression of the disease.
- Treatment with multiple attempts of controlled ovulation induction was done following the diagnosis.
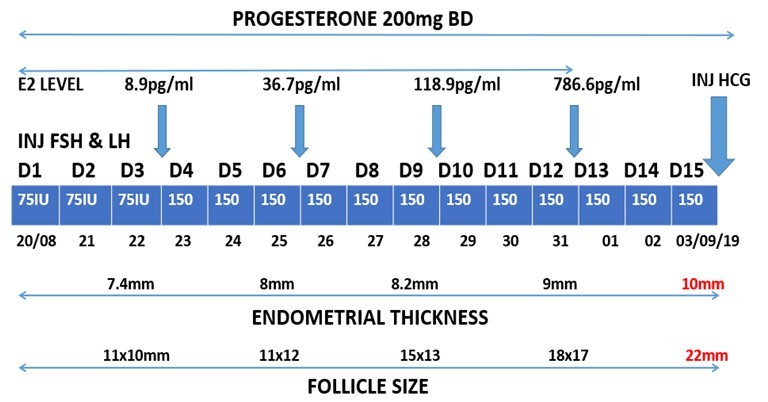
On 05/10/2019, a G-sac of 6 weeks 5 days was noted, she was monitored throughout her pregnancy, and at 5 months POG she was diagnosed with HDP. The patient delivered at 33 weeks POG on 10/04/2020, due to uncontrolled Blood pressure. A preterm baby BOY of birth weight 2.3kgs.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Shreyasi Karmakar is an M.S. (Obstetrics and Gynaecology) practising as an assistant professor in ESI-PGIMSR, ESIC Medical College, Joka, Kolkata.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries