Immunotherapy in Colorectal Cancer
M3 India Newsdesk Dec 27, 2023
This article highlights the promise of immunotherapy in treating a subset of colorectal cancers (dMMR/MSI-H), explaining how immune checkpoint inhibitors like PD-1 blockers have shown efficacy.
Metastatic Colorectal cancer (mCRC) has a median overall survival (OS) of approximately 30 months despite the use of a first-line treatment regimen combining chemotherapy and molecular-targeted agents that inhibit vascular endothelial growth factor (VEGF; e.g. bevacizumab especially for right-sided tumours) or epidermal growth factor receptor (EGFR; e.g., cetuximab or panitumumab especially for left-sided tumour). [1]. Therefore, there is an unmet need for newer therapies that can prolong the survival of such patients.
CRC Classification
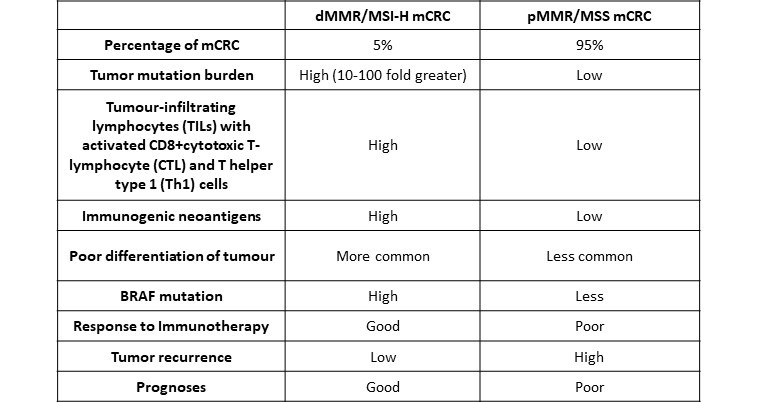
From the anti-tumour immunogenic perspective, mCRC can be categorised into two types of tumours.
- The first type is deficient mismatch repair (dMMR) or high level of microsatellite Instability (MSI-H).
- The second type involves proficient mismatch repair (pMMR) or microsatellite stable (MSS) [2].
Tumour Variations
- dMMR/MSI-H (5%): Responsive to immunotherapy
- pMMR/MSS (95%): Limited response to current immunotherapy
DNA instability and tumorigenesis
- Microsatellites are short segments of the DNA, one to six or more base pair in length, which repeats multiple times in succession and are most often located in the non-coding part of the genome.
- Microsatellites are prone to errors during DNA replication caused by DNA polymerase, resulting in microsatellite instability (MSI-H). Since the DNA mismatch repair (MMR) system plays a key role in recognising and correcting these errors, deficient mismatch repair (dMMR) has very high levels of DNA microsatellite instability as it allows the increased accumulation of frameshift somatic mutation.
- Most MSI-H CRCs are sporadic tumours due to the epigenetic silencing of MMR genes. For patients with Lynch syndrome, MSI-H CRC can be inherited due to germline mutations in MMR genes.

Table 1 – Differences between proficient mismatch repair -microsatellite stable (pMMR/MSS) and deficient mismatch repair microsatellite instability-high (dMMR/MSI-H) colorectal cancer
Immune Checkpoint Inhibitors (ICIs)
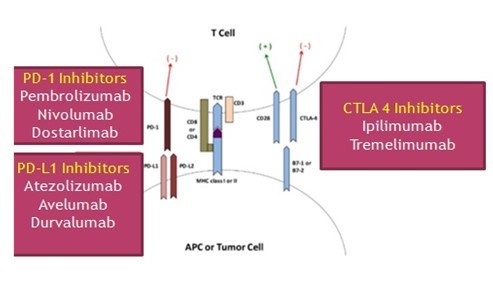
- The immune system plays a key role in eliminating tumour cells. However, MSI-H tumours have upregulation of inhibitory immune checkpoint molecules such as anti-cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), anti-programmed death-1 (PD-1) and anti-programmed cell death ligand 1 (PD-L1) which allow tumours to escape from immune surveillance.
- Immune checkpoint inhibitors (ICIs) block these immune escaping checkpoints and knock down tumour cell defense mechanisms leading to the destruction of tumour cells by anti anti-tumour immune response.
- CTLA-4 is an inhibitory receptor exclusively expressed on T-cells and acts as a negative regulator of the initial priming of T-cells as it outcompetes CD28 in binding to critical costimulatory molecules (CD80 and CD86), which are located on antigen-presenting cells (APCs) [4]. Blockade of CTLA 4 by monoclonal antibodies (Ipilimumab, Tremelimumab) can restore the immune functions of the T-cells.
- The PD-1 receptor acts as a dominant-negative regulator of anti-tumour T-cell effector functioning, by engaging PD-L1 [5]. PD-L1 is, generally, undetectable in normal cells, but inflammatory cytokines, particularly interferon-gamma, stimulate PD-L1 expression on various cell types in the tumour microenvironment. PD-1/ PD-L1 binding inhibits T-cell function, so inhibitors of PD1 (Pembrolizumab, Nivolumab, Dostarlimab) and PD–L1 (Atezolizumab, Avelumab, Durvalumab) can block this T- cell inhibition and restore the anti-tumour immune functions.
Approved therapies and impact
- PD-1blockers pembrolizumab and nivolumab were approved in 2017 by the United States Food and Drug Administration (FDA) for use in MSI-high and dMMR advanced CRC as the second-line treatment for patients who have progressed through first-line chemotherapy [6,7].
- Later pembrolizumab was approved as the first-line treatment of patients with dMMR/MSI-H mCRC in 2020 over standard chemotherapy due to its superiority with respect to clinically significant improvement in progression-free survival (PFS) and favourable treatment-related adverse events (AEs) [8].
- Pembrolizumab had a convenient administration protocol (administered as a 1-h infusion every 3 weeks) and favourable toxicity profiles (mostly immune-related AE, Grade 3-5 AEs in only 22%) whereas chemotherapy had a more complex administration schedule with high rates (66%) of Grade 3-5 treatment-related AEs.
Combination therapies for enhanced response
- Combination of the ICIs such as CTLA-4 blockade by ipilimumab which acts early in the immune response process by inhibiting T-cell activation and PD-1 blockade by nivolumab which acts in later stages by turning off anti-tumour T-cell responses, can act synergistically to promote an anti-tumour immune response.
- Nivolumab plus ipilimumab combination therapy for dMMR/MSIH mCRC was approved in 2018 by the FDA for patients who have progressed after therapy with fluoropyrimidines plus irinotecan or oxaliplatin [9]. This combination has also been tried in the first-line setting with promising results [9].
Immune Check Point Inhibitors (ICIs) in Neoadjuvant setting and positive outcomes
- Nivolumab plus ipilimumab combination was evaluated in a NICHE study in a neoadjuvant setting, in patients with dMMR or pMMR tumours, the pathological response rate was 100% in 20 patients with dMMR and 27% in 15 patients with pMMR.
- In a recent study, PD 1 inhibitor Dostarlimab (500mg intravenous every 3 weeks for 6 months that is 9 cycles) was used in 12 patients with stage II or III dMMR/MSI-H rectal adenocarcinoma as neoadjuvant chemotherapy and all 12 patients showed clinical complete response with no AEs of grade 3 or higher reported [10].
Exploring additional immunotherapy avenues
- Other inhibitory immune checkpoint on T –T-cells includes T-cell immunoglobulin mucin receptor 3(TIM3), T-cell immunoreceptor with Ig and ITIM domains (TIGIT), and lymphocyte activation gene 3 protein (LAG3).
- Blockade of this inhibitory immune checkpoints my monoclonal antibodies (like anti-TIGIT monoclonal antibody tiragolumab) may further activate anti-tumour immunity and is under trials for various tumours.
Strategies for immunologically 'Cold' tumours
Most mCRC (up to 95%) are immunologically cold (low mutation burden, low immunogenicity and low tumour infiltrating lymphocytes) and fail to respond to ICIs, therefore several treatment strategies have been examined to turn these tumours immunologically hot by combining ICIs with other immune-modulating treatments, including angiogenetic inhibitors (anti-VEGF antibody bevacizumab), anti- Epidermal growth factor receptor (cetuximab), molecular-targeted agents (angiogenic and oncogenic kinases inhibitor Regorafenib), chemotherapy and radiotherapy.
Future prospects
- ICI-based immunotherapy has revolutionised anti-tumour treatment in various tumour types in recent years. However, this approach is currently available for patients with dMMR/ MSI-H mCRC, who represent only 5% of mCRCs.
- The most urgent need is developing effective immunotherapy for patients with proficient mismatch repair (pMMR)/microsatellite stable (MSS) cancer, which comprises 95% of mCRC cases.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Vishal Bodh is an Associate Professor, in the Department of Gastroenterology at AIMSS-Chamiana, Shimla.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries