Hypothyroidism in pregnancy: Dr. SK Wangnoo & Dr. Monika Goyal
M3 India Newsdesk Sep 03, 2020
Dr. SK Wangnoo and Dr. Monika Goyal write on hypothyroidism in pregnancy, discussing effects on both untreated mother as well as the foetus/neonate, treatment and postpartum thyroiditis.
For our comprehensive coverage and latest updates on COVID-19 click here.
Pregnancy is a state of physiological stress for both mother and foetus. Co-existing hypothyroidism can add on to the stress and is associated with worse maternal and foetal outcome. Hypothyroidism, both overt and subclinical especially in anti-TPO antibody positive women pose increase risk of miscarriage, preterm delivery, while in foetus may lead to low IQ and attention deficit hyperactivity syndrome.
Disease burden
The total prevalence of hypothyroidism is nearly around 2.5% as per western literature. During first trimester approximately 1 in10 pregnant women develop antibody to TPO and thyroglobulin and hypothyroidism develop in 16% of such patients. In the Indian population, the prevalence appears to be around 0.3 to 0.5% for overt hypothyroidism and 2 to 3% for subclinical hypothyroidism.
Aetiology
Autoimmune hypothyroidism is the most common cause of hypothyroidismduring pregnancy in iodine sufficient areas. Other causes include radio-iodine ablation, surgery for thyroid, central hypothyroidism, effect of drugs like rifampicin and phenytoin.
Physiological changes in the mother
Pregnancy is associated with various physiological changes in thyroid to cope with metabolic stress that can be summarised in the following table.
Physiological changes in pregnancy that influence thyroid function test from Brent 1997
| Physiological change | Thyroid function test change |
| ↑ Thyroid binding globulin | ↑ Serum total T4 and T3 concentration |
| First trimester hCG elevation | ↑ Free T4 and ↓TSH |
| ↑ Plasma volume | ↑ T4 and T3 pool size |
| ↑ Type III 5-deiodinase (inner ring deiodination) due to increased hormonal placental mass | ↑ T4 and T 3 degradation resulting in requirement for increased production |
| Thyroid enlargement (in some women 15%) | ↓ Serum thyroglobulin |
| ↑ Iodine clearance | ↓ Hormone production in iodine deficient areas |
Increase in serum hCG during pregnancy, which has intrinsic thyrotrophic activity peaking at 10 to 12 weeks, increases T3 and T4 but decreases TSH, though it gets readjusted in 2nd and 3rd trimester. As a consequence, the cut off to determine hypothyroidism in pregnancy is different than non-pregnant state and targeted TSH too are lower in pregnant versus non-pregnant state with <2.5 mIU in the first trimester (T1) and <3 mIU in second and third trimester (T2, T3) respectively.
Physiological changes in foetus
Development of thyroid gland starts around 7 weeks as an out pouch from the anterior pharyngeal wall in the midline and gains significant hormone synthesising function around 20-week gestation. Serum T4 reaches adult level around 36 weeks of gestation, though T3 remains low due to high placental deiodinase type 3 activity except in foetal brain where type 2 deiodinase is more functional and helps in maintaining adequate T3 level in brain. Anti-TPO antibody, anti-TG and thyroid binding globulin II can cross the placenta freely and may cause neonatal hypothyroidism or hyperthyroidism.
Effect of untreated hypothyroidism mother
Studies show that untreated hypothyroidism is associated with decreased fertility, increased risk of abortion, increased risk of gestational hypertension, anaemia, abruptio placenta, post-partum haemorrhage, greater risk of overt hypothyroidism especially in anti-TPO antibody positive mother.
Effect on foetus and neonate
Babies born to hypothyroid mother are found with preterm birth, low birth weight, increased risk of respiratory distress. It affects foetal brain development leading to impairment of neuropsychological development. They have learning disability and low IQ. Studies have shown that babies born to overt hypothyroid mothers have IQ score 7 point below mean IQ of children born to mothers who were either euthyroid or were on replacement therapy with LT4. The IQ is even worse if there is coexisting iodine deficiency.
Diagnosis
It is done by measuring thyroid function test including TSH, T3, and T4. Multiple factors can affect TSH levels during pregnancy, apart from various physiological changes that occur during pregnancy, including iodine intake, TPO positivity, BMI, racial and ethnic group. It is because of these reasons that ATA recommends that whenever possible, population–based trimester–specific reference range for serum TSH should be used. In places where population based–trimester specific TSH value is not available, the lower reference range should be reduced by 0.4 mIU/l, while upper reference range should be reduced by approximately 0.5 mIU/l in the first trimester, which corresponds to upper reference range of 4 mIU/l. This reference value should be applied beginning with the late first trimester, weeks 7 to 12, with gradual return towards the non-pregnant range in the second and third trimesters. Whereas, pregnancy specific normal TSH as per European and US based data is <2.5 mIU/l, in first trimester, <3 mIU/l in second and third trimester.
As far as measurement of T3, T4 is concerned, the accuracy FT4, FT4 is not reliable during pregnancy when done by indirect analog immunoassay. Measurement by LC/MS appears to be gold standard but is very costly and difficult to access. When used, trimester specific values need to be used. Instead, ATA suggests measuring TT4 and adjusting it for pregnancy by multiplying by 1.5 to be a more reliable method.
ATA has suggested that all women who are hypothyroid should be tested for anti-TPO antibody. Studies show anti-TPO or anti-Tg thyroid autoantibodies are present in 2% to 17% of unselected pregnant women. Also, in an anti-TPO-positive euthyroid woman, TSH levels increase as gestation progresses, from mean of 1.7 mIU/l (12th week) to 3.5 mIU/l (term), with 19% of women having a suparnormal TSH value at delivery. Because the risk of TSH elevation is increased in this population, increased surveillance of thyroid ab-positive women should occur.
Screening
Universal screening of healthy women for thyroid dysfunction before pregnancy is not recommended. However, caregivers should identify individuals at 'high risk' for thyroid illness on the basis of their medical history, physical exam, or prior biochemical data. When such individuals are identified, prenatal measurement of serum TSH is recommended. If it is above 2.5 mIU/liter, the test should be confirmed by repeat assay. Although no randomised controlled trials are available to guide a response, the endocrine society, believes it is appropriate to give low-dose T4 treatment to bring TSH below 2.5 mIU/liter. This treatment can be discontinued if the woman does not become hypothyroid pregnant or postpartum.
The high risk women include:
- Women over the age of 30 years
- Women with a family history or autoimmune thyroid disease or hypothyroidism
- Women with goitre
- Women with thyroid antibodies, primarily thyroid peroxidase antibodies
- Women with symptoms or clinical signs suggestive of thyroid hypofunction
- Women with type 1 DM or other autoimmune disorders
- Women with infertility
- Women with a prior history of miscarriage or preterm delivery
- Women with prior therapeutic head or neck irradiation or prior thyroid surgery
- Women living in a region with presumed iodine deficiency
- Women currently receiving levothyroxine replacement
Some societies recommend neither for nor against universal screening of all pregnant women for TSH abnormalities at the time of their first visit. They support aggressive case finding to identify and test high-risk women for elevated TSH concentrations by the ninth week or at the time of their first visit before and during pregnancy, and they recognise that in some situations ascertainment of the individual's risk status may not be feasible. In such cases, and where the local practice environment is appropriate, testing of all women by week 9 of pregnancy or at the first prenatal visit is reasonable.
Treatment
The consensus is clear on treating all patients with TSH above pregnancy-population specific upper reference range or TSH >4 mIU/l, in patients with TSH below upper reference range but above pregnancy specific normal TSH value ATA guidelines recommend that anti-TPO antibody positive women should be treated with low dose of 25 to 50 mcg while anti-TPO ab-negative patient should not be treated.
Algorithm for the management of hypothyroidism in pregnancy as American Thyroid Association
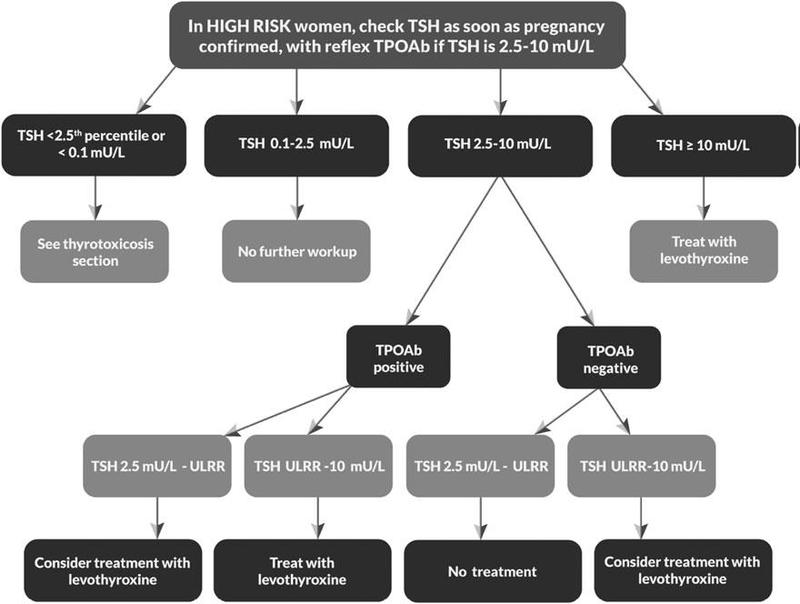
Follow-up
These patients should be followed-up every 4 to 6 weeks and accordingly dose of T4 should be adjusted to achieve targeted TSH in the lower half of the trimester-specific reference range. When this is not available, it is reasonable to target maternal TSH concentrations below 2.5 mIU/L in first trimester and below 3 mIU/L in second and third trimester. Usually, the requirement during pregnancy is 30% more as compared to non-pregnant state. After delivery most hypothyroid women need to decrease the dose of Lt4 to pre-pregnancy level.
Iodine replacement
Iodine deficiency can worsen underlying thyroid dysfunction. To maintain normal thyroid function test, importance should be given to adequate iodine intake both during pregnancy and breast feeding. the recommended average iodine intake is 150 µg/dl in preconception period and 250 µg/dl during pregnancy and breastfeeding. Diet intake should not exceed >500 µg/dl. Although not recommended, measuring urinary iodine concentration may help in assessing adequacy of iodine intake and ideally should be between the range of 150-250 µg/dl.
Postpartum thyroiditis
Women who have anti-TPO antibody hypothyroidism are at increased risk of post-partum thyroiditis, and it is recommended to measure thyroid function at 6 weeks and subsequently at 6-month post-partum period. Women who develop post-partum thyroiditis are subsequently at risk of permanent hypothyroidism and would then need lifelong thyroid therapy.
Finally, hypothyroidism in pregnancy is quite common. All high-risk females and women belonging to at-risk population should be screened for thyroid dysfunction preconceptually. When diagnosed appropriate and timely treatment should be initiated as it is associated with improved outcomes both in mother and baby.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The writer, Dr. Subhash Kumar Wangnoo is a Senior Consultant Endocrinologist and Diabetologist at Apollo Centre for Obesity, Diabetes and Endocrinology (ACODE) in New Delhi.
Dr. Monika Goyal is an Endocrine Fellow.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries