'How to identify patients for Immune Checkpoint Inhibitor (ICI) therapy?': Dr. Anurag Mehta & Dr. Garima Gupta
M3 India Newsdesk Jan 25, 2019
Having established that ICIs are useful for cancer treatment in prolonging progression-free survival as well as providing objective responses better than the current standard of care; the next question is who will be benefited by ICI therapy? How shall the patient be selected?
Selection of patient for ICI therapy
Currently, three biomarkers are being used to select patients for treatment by ICIs:
- Microsatellite Instability high (MSI-H) or deficient in mismatch repair protein (dMMR) tumours
- They are the most well-established of the three for which Pembrolizumab has received FDA approval irrespective of the tumour type and is, therefore, a true agnostic predictive biomarker.
- The constraint of using dMMR is lack of a companion assay with defined antibody clone, Ag retrieval system and labelling method. Likewise, FDA has not approved the kit or microsatellite assay that needs to be used for patient selection.
- PDL1 expressing tumour or immune cells
- PDL1 expression on tumour cells and the immune cells in the microenvironment is the other biomarker that has been used in several trials of ICIs with each trial defining its own method of evaluation, use of antibody clone and cut point to delineate positive from negative.
- Further, disease stage, the line of treatment in which ICIs is being administered are the other variables to be taken into account for positive
- selection of patients.
- PDL1 expression as a predictive biomarker thus is full of confounding mutable. Meta-analysis of 8 studies, keynote 006 & 045, checkmate 017; 025; 057 & 141 and OAK & POPLAR have shown 34% decrease of death with study defined PDL1 positivity but also 20% decrease of death with study defined PDL1 negativity.
- PDL1 expression thus has poor PPV and NPV. PDL1 immunostaining, therefore, is not a robust marker for patient selection for routine PD1 or PDL1 blockade.
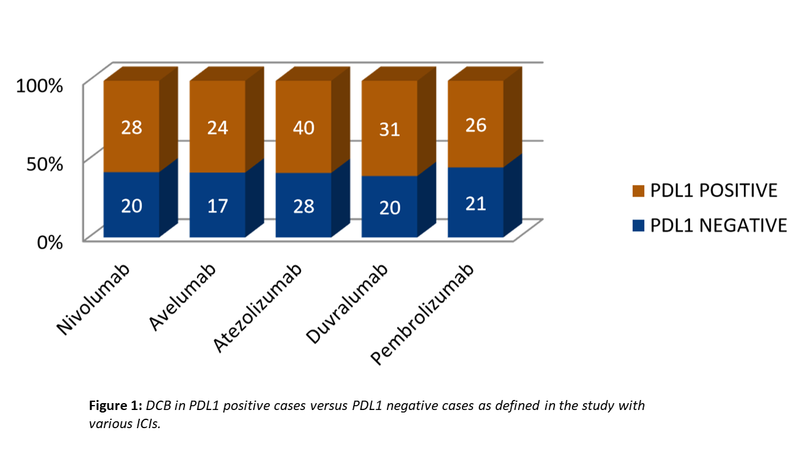
- However, trials with all the drugs have shown superior benefit with PDL1 positive cases vis-à-vis with PDL1 negative cases (Fig. 1). Not fully satisfactory, yet PDL1 expression is a necessary evil that cannot be wished away as yet.
- Tumour Mutation Burden (TMB)
- The last of the three biomarkers being increasingly used in patient selection for ICIs is the “Tumor Mutation Burden” which refers to non-synonymous, non-germline, non-driver and non-SNP somatic mutation load carried by the tumour.
- The philosophy behind measuring TMB stems from the belief that higher the TMB more likely is the chance of tumour possessing unique neoantigens that are likely to be identified as non-self.
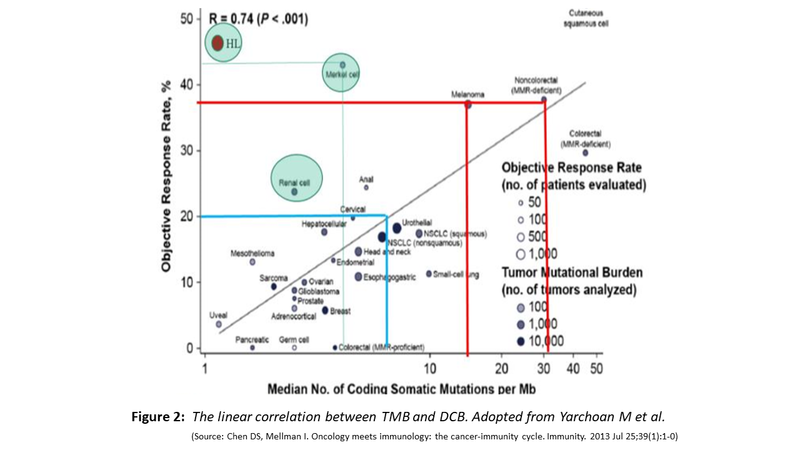
- Shown below (Fig. 2) is the increasing likelihood of obtaining DCB with increasing TMB, exception being RCC, Hodgkin Lymphoma and Merkel cell carcinoma.
- The RCC is capable of producing neoantigens because it is one tumour with highest levels of indels, Hodgkin Lymphoma for reason of 9p24 amplification which amplifies the PDL1 and Merkel cell carcinoma being caused by oncogenic polyomavirus with several virus defined antigens.
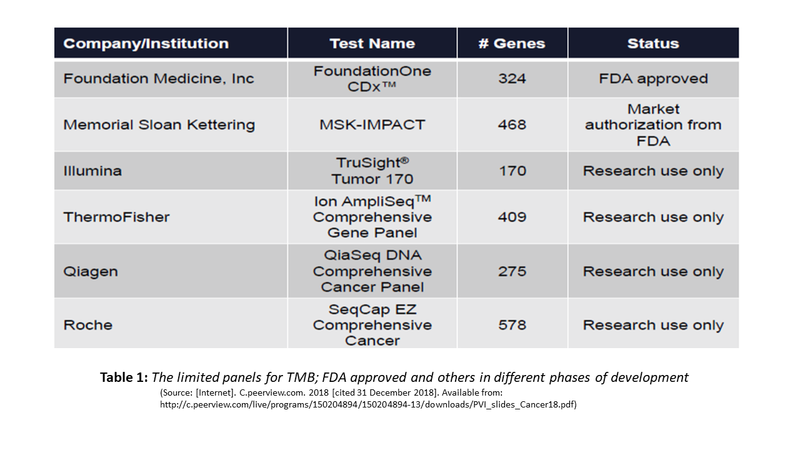
Originally, TMB was assayed using Whole Exome Sequencing (WES). Needless to say that such extended testing with long turnaround time and high cost cannot be an effective biomarker. Currently, shorter versions with a limited panel of genes have become the standard of testing TMB. Two TMB panels have received different levels of FDA approvals and several others are in different stages of validation. The table below exemplifies some of these:
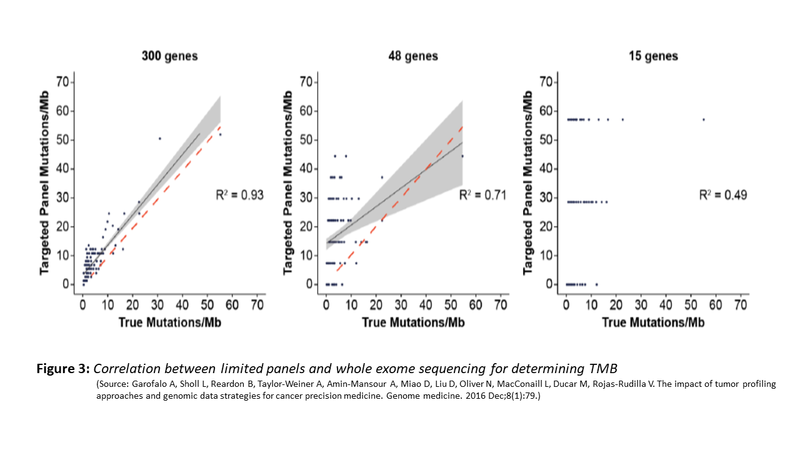
Do these panels correlate with mutation burden identified by WES? The evidence supports the use of such limited panel for TMB. The figure (Fig. 3) below shows the correlation between limited panel and WES. However, a panel size of .5MB is the minimal target size that is needed for such concordance and adequate sensitivity and specificity to achieve.
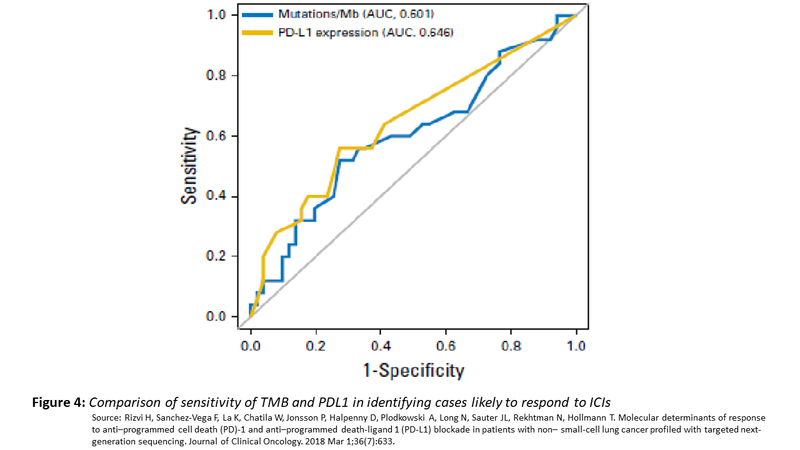
Despite a lot of attention on TMB as a predictive marker for ICIs, the predictive value of TMB is no better than PDL1 expression with the area under curve being similar. Moreover, what value of TMB should be used as a cut point to segregate positive and negative have not been well defined.
Limitations of TMB
- To surmise; TMB like PDL1 expression assay has low PPV & NPV, The DCB with TMB as a biomarker is no better than PDL1.
- TMB is an expensive biomarker, cut off for robust prediction has not been identified either as a general biomarker or cancer-specific marker.
- Further, there is no benefit in combining MSI with TMB (many labs offer the test). MSI-H tumours will always have high TMB but the reverse is not true and their lies the importance of TMB.
- Role of TMB in cancers like GB, Breast, Thymic cancer is undetermined. Targeted NGS is a reasonable substitute to WES for differentiating high TMB vs. low TMB but a panel size of minimum 0.5 MB should be used.
Other strategies
Given the limitations of PDL1 and TMB estimation in terms of their performance characteristics, several additional strategies are being used. To enumerate some of these
- Combining TMB with PDL1 evaluation has shown improvement in PPV of the test
- Somatic mutations in specific genes like the gain of function in KRAS, loss of function in TP53, the gain of function JAK3 and some specific mutations in PBRM1 and SMARCA4 enhance the likelihood of response to ICIs while genetic alterations in EGFR, MDM2, MDM4 and DNTM3A are negative selectors of treatment with ICIs
- Clonality– T cell oligoclonality indicate the likelihood of response as this indicates the available population of T cells reacting to Tumor Antigens
- Neoantigen prediction based upon tumour mutational profile and computational prediction of peptide alterations capable of being immunogenic can enhance the likelihood of response
- Identifying HLA class1 diversity in tumour tissue can also add value to TMB or PDL1 evaluation
- Baseline density of CD8+, CD3+ cells in tumour microenvironment has also been shown to improve outcome with ICIs
Immunotherapy is here to stay as it has already demonstrated DCB. Knowing the basis of immunotherapy can help understand how it functions and provide better insight into harnessing it even further. Current application of biomarkers to identify the patient population that can benefit by this therapy is unsatisfactory but is a work in progress and shall soon solidify to provide a higher level of evidence and better and more efficacious usage of Immunotherapy especially with ICIs.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
Dr. Anurag Mehta, a senior pathologist with more than 30 years of experience in the field, and special interest in molecular biology and diagnostics is also the Director - Department of Laboratory & Transfusion Services and Director Research, Rajiv Gandhi Cancer Institute, N Delhi.
Dr. Garima Gupta is a Senior Research Officer at Rajiv Gandhi Cancer Institute, N Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries