Emerging Therapies for Hepatitis B
M3 India Newsdesk Oct 25, 2023
The article discusses the challenges in treating chronic hepatitis B, the limitations of current therapies, and potential new approaches including immunotherapy, and therapeutic vaccines, all aimed at achieving a functional cure for the disease.
Over 257 million people are chronically infected with hepatitis B virus worldwide, 39 million of whom are in India. The current standard of care for the treatment of hepatitis B including nucleoside/tide (NUCS) analogues and pegylated interferon α (Peg-IFN α), achieves functional cure in only a small proportion of patients.
The currently available therapies are unable to achieve a complete or sterilising cure, due to the ability of HBV to form covalently closed circular DNA (cccDNA) that exists as a mini chromosome within the infected hepatocyte, as well as its ability to integrate within the host genome. So the realistic goal with current therapy for chronic hepatitis B is to achieve a state of ‘functional cure’.
Functional cure (FC) refers to undetectable serum HBV DNA and durable HBs Ag loss, with or without anti Hepatitis B surface antibody seroconversion, after treatment withdrawal. Since cccDNA and integrated HBV DNA persist within infected hepatocytes, the risk of HBV reactivation persists even after FC. The clearance of hepatitis B surface antigen, which is the hallmark of functional cure, is achieved in very few patients. So there is an unmet need for new and more effective drugs, to achieve functional cures.
The new antiviral agents can be broadly classified into two groups:
1. Direct-acting antiviral agents (DAA) that target specific steps in the viral life cycle.
2. Immunomodulators that act on innate and adaptive immune responses against HBV infection.
Direct-acting antiviral agents (DAA)
Recapitulating the HBV lifecycle helps to identify sites that can be targeted by new direct-acting antiviral agents (DAA):
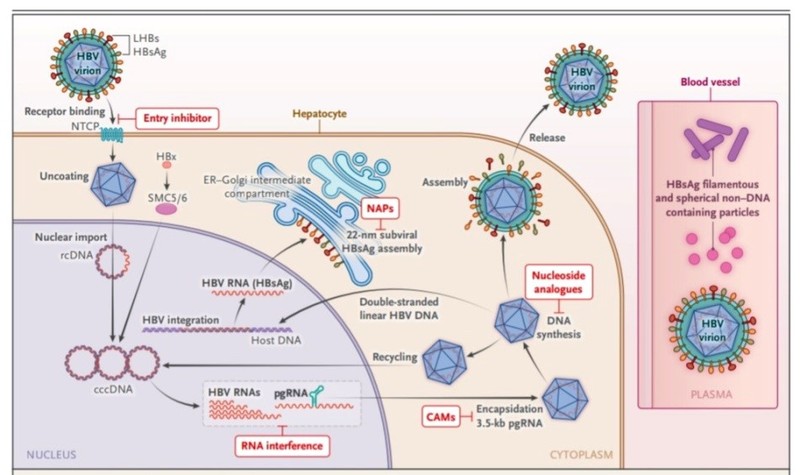 The life cycle of HBV and site of action of new DAA
The life cycle of HBV and site of action of new DAA
The infectious virion attaches by binding the pre-S1 domain of the large HBV surface protein (LHBs) to the hepatocyte entry receptor sodium taurocholate cotransporting polypeptide (NTCP).
Bulevirtide (Hepcludex or Myrcludex)
- It is a synthetic pre-S1 lipopeptide that binds irreversibly to NTCP inhibiting entry of the HBV (and hepatitis D virus) into the hepatocyte.
- The role of Bulevitide in the treatment of chronic hepatitis B is undefined at present; however, the drug was approved by EMA (European Medical Agency) for treating hepatitis D virus infection in 2020.
- After the virus enters the liver cell, it undergoes uncoating releasing nucleocapsids that contain partially double-stranded relaxed circular DNA (rcDNA) and viral polymerase.
- These capsid contents enter the nucleus of hepatocytes through nuclear pores. Within the hepatocyte nucleus, viral polymerase elongates the positive strand of rcDNA to form fully double-stranded covalently closed circular DNA (cccDNA) minichromosome and a virion double-stranded linear DNA segment also integrates into host DNA to form integrated DNA.
- This cccDNA mini-chromosome serves as a template for pregenomic RNA (pgRNA) and subgenomic messenger RNAa (mRNAs) while integrated HBV-DNA serve as templates for subgenomic messenger RNA (mRNAs), so there are dual sources of HBsAg from cccDNA and integrated HBV DNA.
- These mRNA transcripts are translated into viral proteins including hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), hepatitis B core antigen (HBcAg), polymerase ect in the cytoplasm using hepatocyte machinery.
RNA – interfering agents, small interfering RNA (siRNA) and antisense oligonucleotide (ASO)
- It degrades the mRNA transcripts from both the cccDNA and integrated DNA thus silencing the genes and blocking viral protein production and pgRNA expression.
- For siRNA lipid nanoparticle (LNP) delivery avoids sequestration during circulation and conjugation to N-acetylgalactosamine facilitates uptake by asialoglycoprotein receptor on hepatocytes.
- The dose-dependent decline in HBsAg level is seen with both agents, even though the end point of functional cure (HBsAg seroclearence) has not been met to date, the reduction in HBsAg level to less than 100 IU/ml was achieved in the majority.
- As viral mRNA transcripts are translated in the hepatocyte cytoplasm, Core protein (Cp) molecules assemble to form an immature nucleocapsid which encloses pgRNA and HBV polymerases.
- As nucleocapsid undergo maturation, pgRNA is reversely transcribed by the HBV polymerase to minus strand DNA which serves as a template for incomplete positive strand DNA to form partially double-stranded rc DNA within mature nucleocapsid.
Capsid assembly modulators (CAMs)
- CAMs are oral small molecules that disrupt the process of nucleocapsid assembly and maturation and at higher doses may inhibit the formation of new cccDNA. CAM-A molecules result in the formation of aberrant capsids that are rapidly degraded. CAM-E molecules result in the formation of normal but empty capsids.
- The inhibition of HBV polymerase by nucleoside/nucleotide analogues (NUCs) is already a first-line therapy for chronic hepatitis B. The newer drugs in this category undergoing trials are Besifovir, Tenofovir exalidex, Pradefovir and Metacavir.
- The mature nucleocapsid enters the endoplasmic reticulum where they are coated with hepatitis B surface antigen to form the Dane particle that is released into the circulation. A population of mature nucleocapsids re-enters the nucleus and serves to maintain the nuclear cccDNA pool.
- During translation, there is exponential overproduction of viral proteins like HBsAg and HBe Ag which enter the endoplasmic reticulum. While a small proportion is utilised in coating the nucleocapsid to form Dane particles, the vast majority of these viral proteins are secreted into the circulation.
- These non-infectious subviral particles (SVP) constitute over 99.9% of circulating viral products and play a major role in producing T-cell exhaustion and perpetuating CHB infection.
Nucleic acid polymers (NAPs)
- NAPs are amphipathic phosphorothioate oligonucleotides that selectively target the assembly and secretion of spherical SVPs, generated from both cccDNA and integrated DNA, effectively blocking the replenishment of HBsAg in the circulation. The initial results from these agents appear promising with a drop in surface antigenemia, however significant ALT flares remain a concern.
- Gene therapy approaches for the elimination of cccDNA include the use of designer nucleases (Zinc finer nucleases, Transcriptional activator–effector nucleases), gene editing tools (CRISPR-Cas9) and epigenetic silencing. The in vivo application of these techniques is still under development.
Immunotherapy for CHB
The immunomodulatory treatments to restore HBV-specific T-cell and B-cell responses are being researched. While immunomodulators such as interferon α and thymosin-α1 have long been in use for treating CHB, newer immunomodulatory agents in various stages of drug development include:
Pattern recognition receptor (PRR) agonists
- Vesatolimod - Toll-like receptor-7 (TLR-7) agonists
- Selgantolimod – TLR 8 agonists
Inarigivir
- Retinoic acid-inducible gene-1 (RIG-1)
- Nucleotide-binding oligomerisation domain-containing protein 2 (NOD2) activator
The above pattern recognition receptor activates innate immune response against virus.
The antibody or small molecule checkpoint inhibitors against PD
- 1 axis (Nivolumab)
- Cytotoxic T- lymphocyte-associated antigen – 4 (CTLA 4) (ipilimumab)
The above inhibitors have had limited initial effects on CHB.
Vaccination
- Vaccination carried out after the HBV infection has become chronic with the intention of activating effective humoral immunity as well as CD 4+ and CD8+ T-cell responses that control or clear the CHB is called therapeutic vaccination.
- Therapeutic vaccines including peptide vaccines, DNA vaccines and viral vector-based vaccines are in development and under trial.
- HBV- specific T- cell therapy to overcome the specific CD8+ T-cell exhaustion in CHB involves infusing patients with autologous T-cells, engineered to either express a canonical HLA class I restricted T-cell receptor (TCR) or a chimeric antigen receptor (CAR) targeting a specific HBV epitope.
- When these newer agents enter the clinical practice the goal of achieving a functional cure for HBV will be within reach.
Disclaimer- The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of M3 India.
About the author of this article: Dr Vishal Bodh is an Associate Professor, in the Department of Gastroenterology at AIMSS-Chamiana, Shimla.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries