DRDO's COVID remedy 2-deoxy-d-glucose (2DG): What are the pros and cons?
M3 India Newsdesk May 25, 2021
On May 8, 2021, the Drug Controller General of India (DCGI) authorised 2-deoxy-d-glucose (2-DG) for emergency use in persons with moderate to severe COVID-19 as clinical trials have demonstrated that the therapy aids in the speedier recovery of hospitalised patients and decreases their need on supplementary oxygen. However, health experts argue that there is little public evidence on the drug's human trials, casting doubt on its usefulness and potential negative effects.
For our comprehensive coverage and latest updates on COVID-19 click here.
2-deoxy-d-glucose (2-DG) was created in collaboration with researchers at the Institute of Nuclear Medicine and Allied Sciences, which is part of the Defence Research and Development Organization (DRDO), and Dr Reddy's Laboratories, a pharmaceutical company.
Clinical trials have demonstrated that this chemical aids in the speedier recovery of hospitalised patients and decreases their need on supplementary oxygen. In COVID patients, a greater number of those treated with 2-DG had RT-PCR negative conversion. The medicine will be extremely beneficial to those who are afflicted with COVID-19.
Research studies of 2-deoxy-D-glucose (2-DG)
In April 2020, during the first wave of the COVID-19 pandemic, scientists from INMAS-DRDO collaborated with the Centre for Cellular and Molecular Biology (CCMB) in Hyderabad to perform research. It was discovered that the 2-deoxy-D-glucose (2-DG) molecule suppresses viral growth and acts efficiently against the SARS-CoV-2 virus.
The Drugs Controller and Central Drugs Standard Control Organization then approved Phase II studies from May to October 2020, which DRDO carried out in collaboration with its industrial partner DRL, Hyderabad. The Phase III trials were approved in November 2020 and took place from December 2020 to April 2021. The studies comprised 270 patients from 27 hospitals in various states. The medicine was licenced for emergency use on May 1, 2021, and Dr Reddy's Labs is producing it.
The medication was shown to be safe in COVID-19 patients and showed a substantial improvement in their recovery in Phase-II studies (including dosage range) conducted from May to October 2020. Six hospitals participated in the Phase IIa clinical study, whereas 11 hospitals participated in the Phase IIb (dose range) research study. A phase-II study including 110 patients was undertaken.
On multiple endpoints, individuals treated with 2-DG had a greater rate of symptomatic cure than those treated with Standard of Care (SoC). When compared to SoC, a considerably more favourable trend (2.5 days difference) in terms of the median time required to achieve normalisation of particular vital signs parameters was observed.
DCGI approved Phase-III clinical studies in November 2020 based on good results. Between December 2020 and March 2021, the Phase-III clinical study enrolled 220 patients at 27 COVID facilities in Delhi, Uttar Pradesh, West Bengal, Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka, and Tamil Nadu. DCGI was presented with full data from a phase-III clinical study. By Day-3, a considerably greater number of patients in the 2-DG arm recovered clinically and got free of supplementary oxygen need (42% vs 31%) compared to the SoC arm, demonstrating an early cessation of oxygen therapy/dependency.
In patients aged 65 years and older, a similar pattern was seen. On May 1, 2021, DCGI approved this medication for Emergency Use as supplementary treatment in individuals with moderate to severe COVID-19. As a generic molecule and counterpart of glucose, it is cheaply manufactured and readily available in the country.
History
2-DG is an altered glucose molecule with anticancer and antiviral properties. 2-Deoxy-d-glucose is a glucose molecule in which the 2-hydroxyl group has been replaced with hydrogen, rendering it incapable of continued glycolysis. As such, it inhibits the synthesis of glucose-6-phosphate from glucose at the phosphoglucoisomerase level in a competitive manner (step 2 of glycolysis). In the majority of cells (with the exception of the liver and kidney), glucose hexokinase phosphorylates 2-deoxyglucose, trapping the resulting 2-deoxyglucose-6-phosphate intracellularly; hence, labelled forms of 2-deoxyglucose serve as an excellent marker for tissue glucose absorption and hexokinase activity.
Numerous malignancies have increased glucose absorption and hexokinase activity. Although 2-DG has been studied since 1956, it has not been authorised for the treatment of any other disorders. It is mostly utilised for diagnostic testing and research purposes at the moment. 2-Deoxyglucose tagged with tritium or carbon-14 has been a common ligand for laboratory study in animal models, where distribution is determined through tissue slicing followed by autoradiography, which is occasionally used in conjunction with conventional or electron microscopy.
2-DG is taken into the cell via the glucose transporter. Thus, cells that take up more glucose, such as tumour cells, also take up more 2-DG. Due to the fact that 2-DG inhibits cell proliferation, its application as a tumour treatment has been suggested, and 2-DG is now undergoing clinical studies. While a recent clinical experiment demonstrated that 2-DG may be tolerated at a dosage of 63 mg/kg/day, the observed cardiac side effects (prolongation of the Q-T interval) at this level and the fact that the malignancy advanced in the majority of patients (66%) put doubt on the reagent's viability for future clinical usage. However, the mechanism through which 2-DG slows cell development is unknown.
The government issued this photograph in a news release to demonstrate that cell cultures grown in the absence of 2-DG had more viral plaques — distinct areas indicating virus-induced cell damage – than those grown with 2-DG. These investigations were carried out at Hyderabad's Centre for Cellular and Molecular Biology.
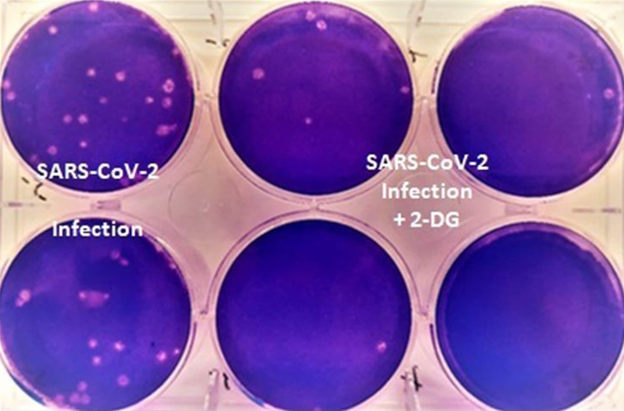
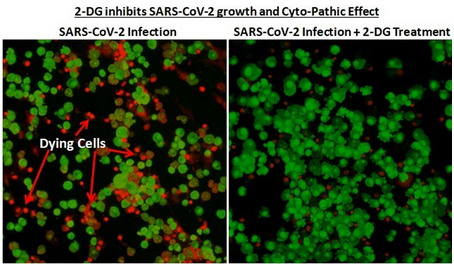
Concerns regarding the use of the drug
As with the approvals of favipiravir, itolizumab, and Verafin by the DCGI, the approval of 2-DG is based on insufficient data. 2-DG is an unlicensed anti-cancer agent that was formerly used to stimulate stomach acid output during influenza testing. It was attempted to treat cancer on its own or in conjunction with chemotherapy. However, health experts argue that there is little public evidence on the drug's human trials, casting doubt on its usefulness and potential negative effects. They believe that without definite phase III study data, the medication may cause damage to healthy cells.
The drug's safety profile was deemed "somewhat dubious." The sample size of 220 patients cited on the ICMR website was insufficient to determine the safety profile in addition to effectiveness. The DRDO and Dr Reddy's performed a phase 3 study of 2-DG in 220 patients across the country. The CTRI registration for this experiment, however, does not specify the factors the study researchers want to evaluate.
For example, the phase 2 protocol states that the key endpoint – the study's major purpose – will be to assess trial participants' progress on a 10-point scale. The secondary endpoint comprises around 15 different variables, including as death, symptom improvement, time spent on supplemental oxygen, and so on. As a result, the study will be considered successful only if the researchers assess a significant positive improvement on the key endpoints — which in the instance of 2-DG were marked on a 10-point scale.
The phase 3 trial's CTRI registration, on the other hand, did not specify what the primary outcomes were. According to the press release, participants in the phase 3 study who received 2-DG had enhanced ‘symptom improvement' and spent less time obtaining supplemental oxygen. But because we don't know if these two characteristics were the major endpoints of the phase 3 study or merely one of several secondary endpoints, we can't say if the study was successful or whether the release is just presenting the study's positive findings. It's also worth noting that the phase 2 trial's secondary objectives were "symptom improvement" and "oxygen dependency."
The claim that 42% of patients on 2-DG improved symptomatically requires further support because 31% did not show any evidence of improvement. We need a thorough review to see whether the improvement, to whatever amount it was, was sustained throughout and whether it genuinely prevented people from being placed on the ventilator.
The following information summarises the properties of 2-deoxy-D-glucose (2-DG):
- The medicine, 2-deoxy-D-glucose (2-DG) is a powdered supplement that comes in a sachet and is used orally by mixing with water. It is like glucose powder and can be taken twice daily with water.
- A COVID-19 patient may require five to seven days of treatment to be entirely treated.
- Because 2DG inhibits viral development, it will also operate against variations. Once development is slowed, experts say, there would be no rapid increase in the body's oxygen consumption.
- The medicine is repurposed since the 2-DG molecule is intended for the treatment of tumours and cancer cells.
- This is a glucose analogue, a substance that has the appearance of glucose but is not glucose. A virus that replicates rapidly in the body requires glucose for sustenance. As a result, the virus will consume this glucose analogue and be halted. After that, the medicine will prevent the virus from replicating.
- Clinical trials have demonstrated that this medicine aids in the recovery of hospitalised patients and decreases their need for supplementary oxygen.
- In COVID patients, a larger number of patients treated with 2-DG had RT-PCR negative conversion.
- 10,000 packages are to be sent to various hospitals throughout India for usage exclusively by hospital medical authorities.
Click here to see references
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author is a practising super specialist from New Delhi.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries