Take the challenge- Solve the case with a Cardiology expert- Part 2
M3 India Newsdesk Oct 21, 2020
Are you ready for part 2 of the cardiology case challenge? Study the next stage covering the findings, clinical examination, lab investigations and possible treatment choices and answer the questions. Get your responses evaluated from the expert.
Type your answers in the boxes below the questions. Professor Sundeep Mishra will share comments as the case progresses. Stay tuned!
For part 1, click here.
For our comprehensive coverage and latest updates on COVID-19 click here.
History
Findings: Adolescent girl with history of progressive dysnoea of recent origin (3 weeks) without any H/O fever or wheezing.
Analysis: Dysnoea in the young can be due to cardiovascular disease or pulmonary disease. The most common causes are asthma, heart failure, pneumonia, bronchiolitis. anaemia, pulmonary thromboembolism, and psychogenic problems.
What is the likelihood here?
- Asthma– Unlikely; dysnoea is progressive over 3 weeks
- Heart failure– Most likely; if cause for progressive dyspnoea can be found
- Pneumonia– Less likely; no fever but pneumonia can happen without fever in immunocompromised individuals and secondly, course over 3 weeks also makes it unlikely
- Bronchiolitis– Unlikely; the child is older (15 years old); bronchiolitis generally occurs in <2 years age
- Anaemia– Possible; if evidence of severe anaemia can be found
- Pulmonary thromboembolism (PTE)– Less likely; no pleuritic chest pain, cough, no evidence of DVT, no predisposing factors (venous stasis, hypercoagulable states, immobilisation, surgery and trauma, malignancy, acute medical illness, etc.)
- Psychogenic– Unlikely; dysnoea is of progressive nature which points towards an organic disease
Possibilities
So after evaluating history we are left with just 3 possibilities:
- Heart Failure– Most likely
- Anaemia– Possible
- Pneumonia, PTE– Less likely
Vital status
Findings: BP- 90/60 mmHg, pulse pressure- 30, PR- 104/minute, weak, RR- 24/min, SpO2- 92% on room air
Analysis: Evidence of shock with narrow pulse pressure; tachypnoea and slight hypoxemia seem secondary to primary cardiac failure (so pulmonary aetiology unlikely). Anaemia as prime cause is also unlikely because of presence of shock.
Possibilities: Heart failure is nearly confirmed ± anaemia TRO PTE or atypical pneumonia
Clinical examination
Salient features are no murmurs auscultable but S3 is present. B/L crepts is present.
Analysis
Presence of S3 confirms heart failure. Absence of murmurs rules out rheumatic heart disease and presence of B/L crepts suggests early pulmonary oedema (left-sided heart failure).
Pulmonary aetiology is ruled out because haemodynamic instability is predominant and out of proportion to respiratory decompensation (tachypnoea and mild desaturation) and no respiratory localisation on examination (no breath sounds or coarse crepts). Second heart sound is not increased.
Cause of heart failure in adolescents
Over-circulation or pump failure can be the cause of heart failure in the young. Common causes include valve diseases, congenital heart disease, myocardial diseases (myocarditis, dilated cardiomyopathy or restrictive cardiomyopathy) or pericardial disease, and severe anaemia.
- Valve disease- Ruled out because there is no murmur; some murmurs may not be auscultable sometimes because of acoustic issues but in this case S3 is ausculable.
- Myocarditis- Likely, because course is suggestive, point against is no fever (but 50% cases of myocarditis happen without fever).
- Congenital heart disease– Some congenital heart diseases like VSD with huge left-right shunt may produce S3 but absence of any murmur, haemodynamic instability and relatively rapid progression makes this diagnosis very unlikely.
- Dilated cardiomyopathy– Possible but progressive worsening over 2 weeks is unlikely unless there is a precipitating factor. Causes could be,
- Ischaemic DCM- This is unlikely because of young age. However, it can occur with ALCAPA or familial hypercholesterolaemia (but again rapid progression is unlikely).
- Drug induced– Ruled out, because no history of intake of anti-cancer or anti-leukaemia drugs
- Rhythm disturbances– Ruled out, because there is no evidence of bradycardia or tachycardia (slight tachycardia is as a compensation to hypotension/shock)
- Muscular dystrophy– Ruled out, because there is no evidence of muscle abnormalities
- Restrictive heart disease– Ruled out, because of predominant left-sided, but no right-sided signs and symptoms, progressive course of disease, presence of S3, pulmonary oedema and shock.
- HOCM– Unlikely, because of rapidly progressive course of disease (without apparent precipitators), pulmonary oedema and shock.
- Pericardial disease– Very unlikely, because of presence of S3 and left-sided failure
- Pericardial tamponade– Points in favour; hypotension and narrow pulse pressure. Points against presence of S3 and left-sided failure almost rule out pericardial tamponade.
- CCP– Practically ruled out because of shock, presence of S3 and left-sided failure instead of right-sided failure
- Severe anaemia– May cause high output failure but in this case, there is narrow pulse pressure with presence of shock.
Possibilities: Heart failure, most likely due to acute myocarditis but could be some kind of DCM. However, can’t rule out PTE or severe anaemia at this stage.
ECG
Findings: Sinus tacychardia (90/min), low voltage QRS, equiphasic axis, P wave ≤2.5 (no P pulmonale). No significant ST-T changes or PR segment deviation.
Analysis
Cause of low voltage QRS and equiphasic axis can be,
- Damping of the electrical signal due to excess air (emphysema, COPD), fluid (pericardial effusion, pericardial constriction, pleural effusion), oedema (anasarca), fat (obesity)
- Electrically inert myocardium due to MI or Chagas disease
- Inflammation/infiltration of the myocardium due to myocardoitis/cardiomyopathy or myxedema
Interpretation: ECG is more important in this case in ruling out rather than ruling in;
- HOCM– Ruled out, because no high precordial QRS voltages, secondary repolarisation abnormalities (ST-segment depression, T wave inversion), left axis deviation, and deep, narrow so-called "needle-like" q waves, typically in leads I, L, V5 and V6 or arrhythmic abnormalities. Abnormal ECG is present in >95% of cases
- Pulmonary embolism is unlikely although sinus tachycardia is present but right heart strain pattern and SIQIIITIII pattern is not present.
- Pericardial disease is also unlikely because although there is sinus tacychardia and low QRS voltage (pericardial tamponade), there is no ST-T (sensitive for pericardial disease) and PR segment depression (specific for pericardial disease) or electrical alternans (pericardial tamponade)
- Severe anaemia (HB 0-5 gm%) is also unlikely because there is no ST segment depression (present in 50-75%), T wave changes (present in 29-50%) or evidence of LVH (present in 25-30%)
Thus, in our context it strengthens the possibility of myocarditis or DCM.
X-ray
Findings: It is an over-penetrated (bedside) film, so not much can be commented but it does reveal presence of cardiomegaly
Interpretation: Again X-ray is more important in ruling out than ruling in;
- Pneumonia– X-ray is investigation of choice for pneumonia and a normal X-ray can practically rule it out
- PTE– A normal X-ray cannot rule out PTE completely (12% of PTE can have it normal), although cardiomegaly is the most frequent finding (38%) and is present, but other signs such as pulmonary infitrates, atelectasis, pleural effusion, pulmonary congestion, elevated hemidiaphragm are not present; also note the more specific signs for PTE; Palla's sign (enlarged right descending pulmonary artery- 87% specificity), Westermark sign (regional oligaemia- 92%) and Hampton's hump (peripheral wedge of airspace opacity- 82%) are absent
- Pericardial effusion/tamponade– While there is evidence of cardiomegaly in pericardial effusion/tamponade the cardio-pericardial silhouette is not symmetrically enlarged (globular or water bottle appearance), and there is no oligemia in lung fields; thus pericardial tamponade is highly unlikely
- CCP– Pericardial calcification (present in 50%) is not seen; also no evidence of pleural effusion or tubercular lung disease; thus X-ray chest per se is not suggestive of CCP
- DCM/myocarditis– Likely, because of cardiomegaly with relatively plethoric lung fields
Other lab investigations
Analysis: Of note is mild anaemia (Hb 9), leucocytosis (TLC 10,500, neutrophil 71%), dyselectrolytemia (hyponatremia and hypokalemia), mild hepatic derangement (OT/PT=151/261), elevated BNP, subclinical hypothyroidism (T4=8; TSH=7.7).
Interpretation
Significantly elevated BNP confirms the diagnosis of decompensated heart failure. There is mild anaemia which cannot be solely responsible for severe clinical condition and haemodynamic abnormality seen here. However, it can contribute to worsening picture.
Likewise, hypothyroidism is subclinical (TSH <10, T4 is normal=8) which alone cannot explain the clinical picture. A condition involving low levels of serum triiodothyronine (T3), known as “low T3 syndrome,” is fairly common in patients with heart failure and indicates that less active hormone is available to the heart through blood plasma.
There is mild hepatic dysfunction which could be a consequence of global hypoperfusion including hepatic hypoperfusion (because of shock) or mild viral hepatitis (and viral myocarditis).
Interpretation: Thus, after considering everything the diagnosis of acute myocarditis, heart failure and shock remains the first possibility (troponin non-elevation is the only odd point but troponin elevation is frequent but not universal in myocarditis) with associated anaemia, sub-clinical hypothyroidism and electrolyte disturbance can be entertained. Anaemia can be true anaemia (likely due to nutritional deficiencies particularly iron deficiency anaemia) but it can also be linked to haemodilution, elevated sympathetic tone, impaired oxygen delivery and myocardial oxygen extraction, and renal dysfunction (here renal functions are normal). Most likely, the cause of myocarditis is viral myocarditis (coxsackievirus group B, human herpes virus 6, and parvovirus B19, echoviruses, Epstein-Barr virus and rubella virus) but bacteria, fungi, parasites can also cause and rarely it can be auto-immune.
Answers to questions from part 1
We received more than 100 responses. Here are the responses as per questions and the discussion.
What is your provisional diagnosis?
65% of respondents correctly made the diagnosis of heart failure.
The features diagnostic of heart failure are as follows:
- Progressive dyspnoea without chest pain
- Haemodynamic compromise out of proportion to respiratory compromise
- Third heart sound is present
- B/L fine crepts
- Cardiomegaly without pulmonary involvement
- Increased DLC with neutrophilia is suggestive of myocarditis
- Elevated BNP is strongly suggestive of heart failure
- Mild hepatic dysfunction could be a consequence of congestion or viral myocarditis
Among the reasons for heart failure following responses were obtained;
1. Pericardial effusion/tamponade was the most common cause suggested for HF- 22%. Features suggesting pericardial tamponade are progressive dyspnoea, haemodynamic compromise, low ORS voltage on ECG and cardiomegaly on X-ray chest. However, several points are against the diagnosis of pericardial effusion/tamponade:
- Presence of LV S3 practically rules out pericardial tamponade
- B/L crepitations are very unlikely
- ECG– No PR segment depression or pulsus alternans seen
- X-ray chest– Cardiomegaly is not-symmetrical/globular with no oligemia
- Raised BNP– Almost rules out Pericardial tamponade
2. Pericarditis– 5%; features against the diagnosis of pericarditis/CCP:
- Progressively increasing dyspnoea as the prime complaint– CCP unlikely
- Left-sided failure > right-sided, signs and symptoms– rules out CCP
- ECG– No PR segment depression, no ST-T changes
- X-ray chest– No pericardial calcification seen
- Raised BNP level makes diagnosis of CCP unlikely
3. Hypothyroidism– Interestingly, 14% thought hypothyroidism could be a cause for the clinical manifestation. Features against the diagnosis of hypothyroidism as the prime cause:
- Hypothyroid diastolic dysfunction is the most frequent cardiac abnormality > pericardial tamponade > systolic dysfunction (with S3) is the least common
- Only mild thyroid involvement– marginally raised TSH 7.7 with normal T4 value of 8. However, generally TSH >10 are associated with significant cardiac abnormalities acutely.
- Heart failure can per se cause an abnormality commonly referred to as “low T3 syndrome”
4. Dilated cardiomyopathy– 6% respondents; while DCM is indeed a possibility there are some factors against it:
- Progressive worsening over 2 weeks without a precipitating cause makes it unlikely
- No ischaemic, or rhythm disturbances identified
- No history of drug intake (anti-cancer or anti-leukaemia)
- No clinical findings of muscle dystrophy
5. Anaemia– 6% respondents; features against the diagnosis of anaemia as the prime cause:
- Relatively rapidly progressive dyspnoea
- Hypotension with narrow pulse pressure (anaemia produces high-output failure with wide pulse pressure)
- Only Hb values <5 can lead to heart failure
6. Respiratory system abnormalities (COPD/cor-pulmonale, pneumothorax, pleural effusion)– 6% respondents; features against primary respiratory disease:
- No cough, rales or coarse crepitations
- Tachypnoea, and hypoxemia present (SpO2- 92%) but haemodynamic compromise; hypotension and tachycardia much more out of proportion suggesting primary cardiac disease
- Second heart sound not increased
- ECG- No right-sided enlargement (ventricle or atria)
- X-ray chest– no parenchymal/pleural lung disease obvious
7. Hypertrophic obstructive cardiomyopathy (HOCM)– 4% respondents; features against HOCM:
- Rapidly progressive dyspnoea without any precipitating factor (arrhythmia, endocarditis) is very unlikely
- No classical symptoms of HOCM present (chest pain, syncope or palpitations)
- Haemodynamic compromise very unlikely unless burnt out HCM
- No S4 or cardiac murmurs auscultated, rather presence of LV S3
- ECG shows no high precordial QRS voltages, ST-segment depression, T wave inversion, left axis deviation (rather equiphasic axis), "needle-like" q waves (leads I, L, V5 and V6) or arrhythmic abnormalities; abnormal ECG is present in >95% of cases
8. Congenital heart disease (CHD)– 4% respondents; features against congenital heart disease:
- Relatively rapid progression of disease without any precipitating factor
- Haemodynamic instability
- Absence of any murmur
- ECG and X-ray chest not suggestive of any CHD
9. Pulmonary thromboembolism (PTE)– 2% respondents; features against PTE:
- No pleuritic chest pain or cough
- No evidence of DVT or predisposing factors for DVT/PTE
- LV S3 present, 2nd heart sound is not raised
- No coarse crepts or rhonchi
- ECG- no right-sided strain pattern or SIQIIITIII pattern
- X-ray chest- Signs of PTE such as pulmonary infitrates, atelectasis, pleural effusion, pulmonary congestion, elevated hemidiaphragm, Palla's sign, Westermark sign or Hampton's hump are not present
10. Right-sided cardiomyopathy (RCMP)– 2% respondents
- Progressive dyspnoea as presenting complaint of RCMP is very unlikely; it generally presents with right-sided signs and symptoms like peripheral oedema
- Haemodynamic compromise is very unlikely, generally terminal
- LVS3 is very unlikely, generally has prominent 4th heart sound
- ECG and X-ray not suggestive of RCMP
Is the patient in shock? If yes, what kind?
- Septic
- Hypovolumic
- Cardiac
- Obstructive
Participants responded as follows:
- Cardiogenic- 63%
- Hypovolumic- 9%
- Obstructive- 13%
- Septic- 1%
- Uncertain- 3%
- No reply- 8%
The patient has shock with narrow pulse pressure. Pulmonary artery occlusive pressure (PCWP) is likely elevated (prescence of B/L crepts). Pulse pressure is broad and pulse bounding in septic shock. PCWP is decreased in hypovolumic shock. Moreover, clinically, the patient has evidence of left ventricular failure (B/L crepts). Thus, the patient has cardiogenic shock.
Further investigations and course
Echocardiography with Doppler: 93% of respondents felt that a proper Echocardiographic examination was mandatory in this case.
Echo revealed:
- Global LV hypokinesia- EF: 15-20 %
- LV size upper limit of normal; LVESD = 43 mm, LVEDD = 50 mm
- Moderate MR– No thickening of mitral valve
- Moderate TR, moderate PAH
- RVSP = RAP +36
- Mild circumferential pericardial effusion; no evidence of tamponade
Analysis of Echo: Echo revealed gross LV systolic dysfunction (EF<20%) but of recent origin (because no significant increase in LV dimensions). Mod MR could be of recent origin due to sudden (but moderate) increase in LV size (over previous) and is not likely to be pathogenic because it is not routinely auscultable, moreover, mitral valve is not thickened (so rheumatic heart disease is not present). Right-sided features seem secondary to left-sided abnormalities (and don’t suggest any RCMP). Mild pericardial effusion may be secondary to overall disease.
Interpretation: Based on relatively acute occurrence of LV systolic dysfunction myocarditis seems the most likely diagnosis. Since LV is not yet dilated, chronic DCM can be ruled out. Likewise pericardial effusion is present but is not significant and therefore cannot explain the overall clinical picture.
How to confirm the diagnosis
Endomyocardial biopsy– 1% of respondents
In the past, endo-myocardial biopsy was confirmatory for diagnosis of myocarditis. EMB usually reveals active or borderline myocarditis with varying degree of fibrosis. More specific kind of myocarditis like eosinophilic myocarditis and giant cell myocarditis can also be diagnosed. However, because of its low sensitivity, low availability, and risk of an invasive procedure in the current era it is no longer the investigation of choice. Not surprisingly only 1% of participants recommended EMB.
Cardiac magnetic resonance imaging– 8% of respondents
It is currently considered as the gold-standard for the diagnosis and follow‑up cases of myocarditis. Moreover, in suspected myocarditis, it can help in the localisation and quantification of tissue injury including oedema, hyperemia and fibrosis. By Lake Louis criteria, the diagnostic accuracy is nearly 78%. It should be carried out in all clinically stable, suspected cases of myocarditis prior to EMB.
Interestingly, only 8% of participants advised this investigation, probably because very few of them suspected myocarditis as a cause for the clinical condition.
The MRI finding in our case
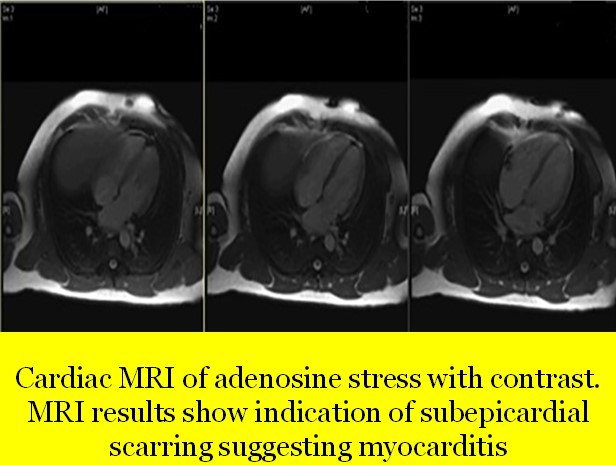
- Left shows mild dilatation, right ventricle is normal in size
- No evidence of myocardial oedema or intra-myocardial iron deposition
- Patchy area of increased signal intensity of myocardium predominantly left ventricle
- Early Gadolinum enhancement with patchy areas
- On delayed enhancement imaging, there was sub-epicardial enhancement in the basal to mid inferior, inferolateral and anterolateral segments, as well as in the apical inferior and lateral segments in a pattern which was most consistent with myocarditis
- Cardiac MRI with adenosine stress with contrast revealed sub-epicardial scarring suggesting myocarditis; there was no evidence of segmental wall motion abnormalities for either ventricle (no ischaemic abnormality)
Thus, cardiac MRI confirmed the diagnosis of myocarditis.
Cardiac biomarkers: 6% participants suggested these tests
Sensitivity of these markers varies depending upon the time of myocardial injury and onset of clinical symptoms, but they may help in the diagnosis of myocarditis. Elevated erythrocyte sedimentation rate (ESR) and C‑reactive protein (CRP) are non-specific lab findings in suspected myocarditis. Elevated cardiac troponin (I and T) are more sensitive than creatinine kinase levels in acute myocarditis. However, normal values do not exclude myocarditis but if increased cardiac troponin levels have been shown to be of prognostic significance.
- Troponin T- At first examination, troponin was 0.15, but in the next examination, troponin was 21.8 ng/mL (maybe, the first report was wrong?)
- CK-MB- 77 ng/mL
- C-reactive protein level- 131 mg/L
Viral/bacterial serology: 4% of participants suggested it
It has very limited utility in the diagnosis of viral myocarditis due to the high prevalence of circulatory immunoglobulin (Ig)G antibodies to cardiotropic viruses in the general population without any evidence of heart disease.
Serology for following viruses was sent; HBV, HIV, EBV, Adenovirus, COVID
- HBV– Negative
- HIV– Negative
- EBV– Negative
- Adenovirus– Negative
- COVID-19– Negative
Viral culture: 0% of respondents
Viruses are difficult to isolate and cultivate. Virus isolation from the blood sample is useful for providing evidence of systemic infection but not for providing information on specific organ disease. Hence, it was not performed.
Ultrasound abdomen: 11% of respondents
USG abdomen was suggested by 11% of participants, perhaps in view of mild derangement of hepatic function as also to differentiate between various types of shock. However, in our case it was rather unremarkable, except for mild pericardial effusion, hypo-contractile heart and mild distention of internal jugular vein and inferior vena-cava.
Respiratory system evaluation: 12% of respondents
Respiratory system evaluation including HRCT and even pulmonary angiogram was suggested by 12% of respondents perhaps with a view to rule out lung parenchymal and pulmonary vascular disease/PTE. However, in view of previous tests clearly establishing the diagnosis of myocarditis, these tests were not performed.
Coronary angiography (CART): 7% of respondents
CART ± cardiac catheterisation was recommended by 7% of respondents but in view of a young girl with no chest pain, no ST-T changes on ECG, no wall motion abnormalities on Echo and stress MRI the possibility of ischaemic cardiomyopathy was ruled out and therefore this test was not performed.
Blood glucose panel: 4% of respondents
Four percent participants recommended the glucose panel perhaps with the view to rule out diabetic cardiomyopathy. However, as discussed earlier, diabetic cardiomyopathy is not a possibility (and therefore not mandatory) but glucose panel was anyway performed (as routine examination)
- Fasting blood sugar– 91 mg%
- HbA1c– 4.1
Autoimmune antibodies: 6% of respondents
Perhaps, in view of the suspected autoimmune nature of the disease as also to look into the aetiology of mild hypothyroidism, 6% respondents recommended ANA and anti-TPO tests.
In our case;
- ANA- 1:40
- TPOAb– 30 IU/ml
Treatment
This is a case of fulminant myocarditis (FM) presenting with severe cardiovascular compromise within 3 weeks since the onset of symptoms. Mortality rates in fulminant myocarditis in children ranges from 20 to 48%. However, this patient is even sicker; presenting with shock leading to global hypoperfusion as manifested by hepatic failure, high BNP suggesting decompensated heart failure and associated comorbidities like anaemia and hypothyroidism. Moreover, already there is dys-electrolytemia; hyponatremia and hypokalemia. Thus, the patient is really very sick and would be difficult to manage.
1. Invasive haemodynamic monitoring: 2% of respondents
In view of the gravity of clinical situation, invasive haemodynamic monitoring was planned. Interestingly, only 25 of the respondents suggested it.
- Radial line was introduced and arterial blood pressure monitoring was instituted.
- Swan ganz catheter was introduced and pulmonary capillary wedge pressure was measured to guide fluid therapy. PCWP was 18 mmHg (also confirming the diagnosis of cardiogenic shock).
- Daily weight and urine volume estimation was also done.
2. Ionotropic support: 50% of respondents
Ionotropic support can vary from simple fluid administration to use of oral ionotropes like digitalis, ACE-I/ARBs, ARNI to IV ionotropes (dopamine, dobutamine, adrenalin, nor-adrenalin, milrinone, vasopressin) to use of cardiac assist devices (IABP, Impella, Tandem Heart, ECMO). Half of all respondents recommended some form of cardiotropic therapy.
- Since PCWP was already 18, IV fluids were not considered.
- IV Dobutamine was instituted at the dose of 0.5 ɥg/kg/min IV continuous infusion initially, increased to 10 ɥg/kg/min (till SBP of 100 mmHg, MAP 65 mmHg and good urine output was achieved)
3. Decongestion, salt and water balance: 65% of respondents
Proper decongestion and maintenance of fluid, electrolyte balance was recommended by nearly 2/3rd of the respondents. Measures include fluid restriction/administration, administration of diuretics (loop, MRA), potassium supplementation or administration of vaptans.
What we did: Since it appeared to be hypervolumic hyponatremia and hypokalemia (dilutional dys-electrolytemia), the following steps were undertaken:
- Fluid restriction– Of up to 1000 ml/d) initially with the aim to maintain wedge pressure ~14 mmHg.
- Potassium supplementation– Oral dose of 2 mmol/kg was administered. Serum potassium repeated after 2 hours revealed a value of 3.3 mEq/L. Another dose of 2 mmol/kg was administered and serum potassium 2 hours later was 3. mEq/L. Serum sodium was by now 136 mEq/L.
- Diuretic– 2 mg/kg IV furosemide was given immediately and repeated after 12 hours, then given B.D with the aim to bring down PCWP to ≤14 mmHg.
- Angiotensin receptor blocker, telmisartan 20 mg OD was given once SBP was >100 mmHg.
- Vasoidilator– Nitroglycerin 10 ɥg/min was also instituted once sbp was >100 mmHg.
4. Maintenance of Ventilation: 23% of respondents
Nearly 1/4th of the respondents felt some sort of ventilator support was necessary. The support can vary from simple oxygen administration with nasal cannula to NIV (non-invasive ventilation) to CPAP/BPAP to full mechanical ventilation.
What we did: Since oxygen saturation was 92% with tachypnoea and accompanied with shock we decided to deliver oxygen by NIV to keep oxygen saturation >95%.
5. Anti-viral therapy: 3% of respondents
Immunotherapies like steroids and IV immunoglobulins may be used in myocarditis. Three percent of our respondents suggested use of steroids. High-dose intravenous immunoglobulin (IVIG) has shown potential in the treatment of myocarditis, perhaps due to its antiviral, antibacterial, and immunosuppressant properties. However, their clinical usefulness still remains controversial. High-dose steroids may promote the recovery of left ventricle systolic function in acute myocarditis but its use in clinical practice is also controversial.
In our patient we gave:
- Immunoglobulin therapy- We employed a high-dose IVIG regimen with a dosage of 2 g/kg for 1 day, and 400 mg/kg/day for 5 days.
- Steroids- We used intravenous prednisolone at >10 mg/kg/day.
6. Thyroid replacement therapy: 6% of respondents
In patients with preexisting heart failure, subclinical hypothyroidism with TSH ≥7 mIU/L and isolated low T3 levels are associated with poor prognosis. On the other hand, treatment with levothyroxine (synthetic T4) in patients with heart failure is associated with a higher risk for all-cause mortality, cardiovascular death, and major adverse cardiac events. The reason is that in patients with heart failure “low T3 syndrome,” is fairly common and the reason for raised TSH is that a less active hormone is available to the heart through blood plasma. When this type of sub-clinical hypothyroidism is treated with levothyroxine, there is often a relatively high thyroxine/T3 ratio in serum with relatively low liothyronine levels in blood, which could further exacerbate the T3-deficient state in patients with chronic heart failure. Thus, although 6% of respondents supported its use, in our patient we did not undertake thyroid replacement therapy.
7. Correction of anaemia: 5% of respondents
Anaemia is a very common comorbidity in patients with heart failure (HF), affecting ~50% patients with acute decompensated HF. Absolute or functional iron deficiency (ID) is seen in ∼50% patients with HF. Correction of anaemia in acute setting has remained controversial. Five percent of our respondents supported the correction. The correction can be undertaken as IV iron replacement, use of erythropoietin or an analogue or even packed cell transfusion. In our patient we did not actively treat anaemia.
8. Beta-blockers: 5% of respondents
Beta-blockers are now drugs of first choice in chronic heart failure. However, their use in decompensated heart failure with shock is controversial and probably not justified. In our patient we did not use any beta-blocker in the acute setting.
This is a special, real-life case challenge series. Send in your responses latest by October 24 (Saturday).
Stay tuned for the next part which will cover the third stage of the case and the expert's discussion of the answers to the questions posted above.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Sundeep Mishra is a Professor of Cardiology.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries