Adjuvant chemotherapy in PDAC- Choosing wisely: Dr. Vivek Agarwala
M3 India Newsdesk Jun 24, 2020
Dr. Vivek Agarwala briefs on adjuvant chemotherapy as a treatment option for exocrine pancreatic ductal adenocarcinoma (PDAC), one of the most challenging cancers for which only surgery was considered as a curative option until now.

Exocrine pancreatic ductal adenocarcinoma (PDAC) is not amongst the most common cancers but remains one of the greatest challenges in Oncology, expected to be the 3rd or 2nd leading cause of cancer-deaths in high-income countries in the next decade. [1] Despite recent advances, survival rates are poor with only 8-11% patients being alive at 5 years after diagnosis. [2]
Surgery is the only potential curative treatment for PDAC but only 10-20% patients at diagnosis are amenable for curative resection. More than 80% patients are metastatic or locally advanced and unresectable at diagnosis after careful staging. [3] Even after curative surgery, the outcomes of surgery alone are dismal with a median survival of 15 to 20 months and a 5-year survival of 8 to 15%, owing to the high frequency (85-95%) of local and distant relapses. [3] Adjuvant chemotherapy, in the last few years, has changed the prognosis of resected PDAC patients, significantly increasing the median overall survival.
Adjuvant story in PDAC has evolved since last 2 decades with multiple treatment options now.
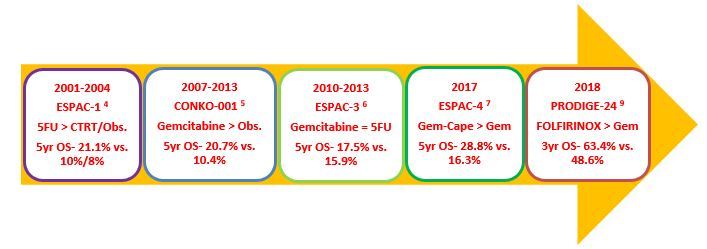
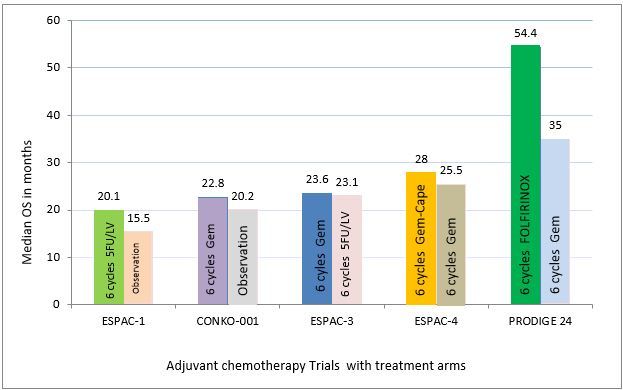
In the early 2000s, the ESPAC-1 study showed that adjuvant chemotherapy with 6 cycles of 5FU/LV improves overall survival (OS) over chemo-radiotherapy or observation. [4] Later, the CONKO-001 study changed the practice towards single agent gemcitabine administered for 6 cycles, 3 weeks out of 4, showing improved median disease-free survival (DFS) (13.4 versus 6.9 months; p < 0.001), compared to observation alone. OS was also significantly improved (5-year OS 20.7% vs. 10.4% and 10-year OS 12.2% vs. 7.7%). [5]
The ESPAC-3 study showed that outcomes are equivalent for 5FU/LV and gemcitabine as adjuvant chemotherapy for resected PDAC. [6] Further, analysis of the study showed that there was no difference in survival between patients commencing chemotherapy within 8 weeks of surgery and those commencing later. Completion of total 6 cycles was also associated with improved survival.
Gemcitabine, due to ease of administration and acceptable toxicity profile, remained as the standard of care for long till 2017-2018.
In 2017, the ESPAC-4 study reported a significant OS benefit with adjuvant gemcitabine plus capecitabine compared with gemcitabine alone, especially in the R0 resected sub-group, with a mild increase in toxicities. However, recurrence-free survival was not significantly different between the arms suggesting differences in post-recurrence survival between the two groups. [7] Further, the CONKO-005 study showed no benefit of adding erlotinib to gemcitabine. [8]
After combination chemotherapy with FOLFIRINOX and gemcitabine + nab-paclitaxel protocols were both found effective in 1st-line metastatic setting; they were both tested in the adjuvant setting too.
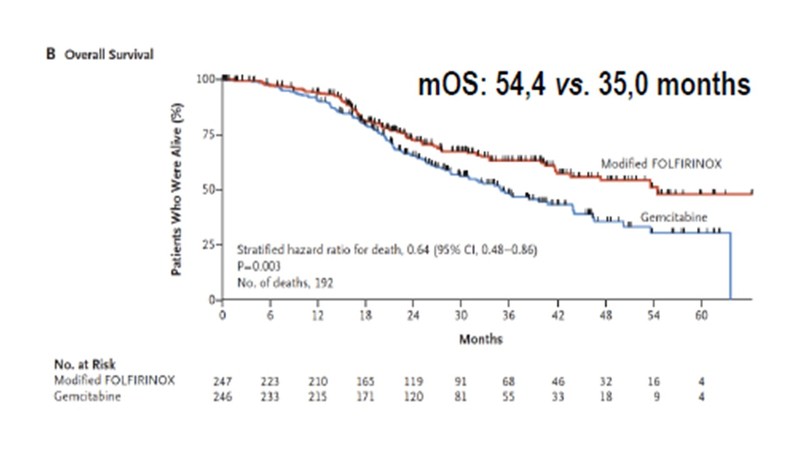
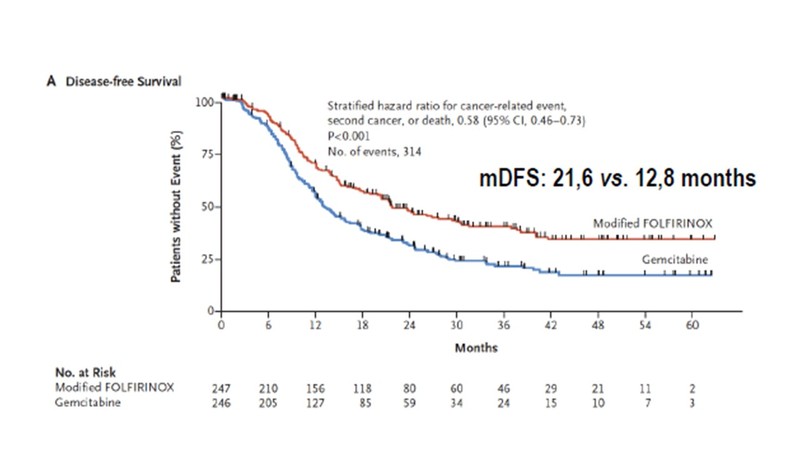
The PRODIGE 24-CCTG PA-26 trial examined 12 cycles of mFOLFIRINOX (modified with no bolus fluorouracil and 150mg/m2 dose of irinotecan) against 6 cycles of gemcitabine monotherapy, recruiting 493 patients of resected PDAC. [9] The primary end point, median DFS was 21.6 months in the mFOLFIRINOX group and 12.8 months in the gemcitabine group (stratified HR, 0.58; 95% CI, 0.46–0.73; p <0.0001). Median OS was 54.4 months vs. 35.0 months (HR, 0.64; 95% CI, 0.48–0.86; p = 0.003). These results are the best DFS and OS data reported so far for an adjuvant treatment of resectable PDAC.
As expected, grade 3–4 toxicities (mainly neutropenia, diarrhea, mucositis and neuropathy) occurred in 75.9% of patients in the mFOLFIRINOX group compared to 52.9% of patients in the gemcitabine group. However the toxicities were manageable, and the rate of grade 4 toxicities was the same for the 2 groups (12%). [9]
The phase III APACT study showed that nab-paclitaxel + gemcitabine combination did not improve the primary end point i.e. DFS, compared to gemcitabine alone (mDFS 19.4 months vs. 18.8 months), while OS was slightly improved (mOS 40.5 months vs. 36.2 months). Grade 3 and 4 hematological toxicities, fatigue and neuropathy was much higher with the combination arm. [10]
Platinum-based chemotherapy, other than FOLFIRINOX, may also confer a survival benefit in patients with resected PDAC and a pathogenic germline BRCA1, BRCA2, or PALB2 mutation. [11] However, this needs to be confirmed in a randomised controlled trial.
The algorithm for treating resectable PDAC is given below:
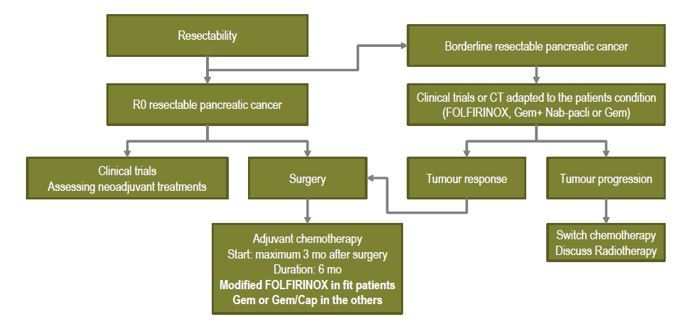
Conclusion
Modified FOLFIRINOX is currently the standard of care for adjuvant chemotherapy in resected PDAC patients who are fit – ECOG PS 0-1, no contraindication to fluropyrimidines, and without severe post-surgical diarrhoea, existing neuropathy or uncontrolled comorbidities. In patients who are not candidates for FOLFIRINOX, gemcitabine-capecitabine combination is another treatment option. In frail and elderly patients, only single agent gemcitabine should be offered.
Click here to see references
Disclaimer- The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of M3 India.
The author, Dr. Vivek Agarwala is a Consultant at the Department of Medical Oncology & Hemato-Oncology at a prominent Kolkata hospital.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries