Diagnostic dilemma- DLBCL vs. MCL: Dr. Vishwanath S & Dr. Govindarajan MJ
M3 India Newsdesk May 12, 2020
Dr. Vishwanath Sathyanarayanan and Dr. Govindarajan MJ detail on an interesting case study of a patient who was initially diagnosed with DLBCL and later with MCL. They quiz on the best treatment approach, role of PET and assessment of treatment response.

This article details a case study and contains questions to help you test yourself on treatment approach for DLBCL and MCL. Explanation for each correct answer has been mentioned below each question.
Case discussion
A 75-year-old gentleman presented with h/o nasal blockade, preauricular and forehead swelling of 2 to 3 weeks duration. He also reported a history of fever, significant weight loss and drenching night sweats. On exam his ECOG performance status was 1 and he had enlarged cervical, axillary and inguinal lymph nodes (LN) with splenomegaly.
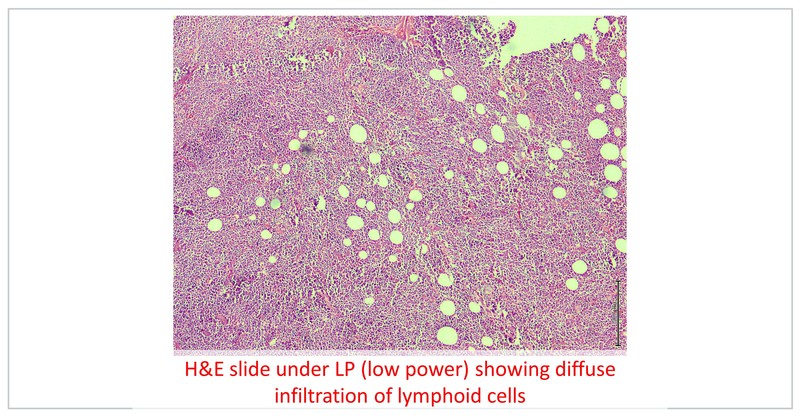
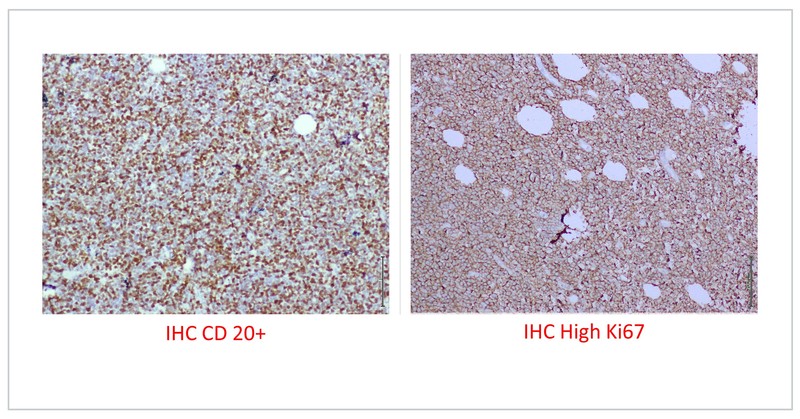
Fine needle aspiration was suggestive of non-Hodgkin lymphoma (NHL). He underwent left pre-auricular LN excision biopsy which was suggestive of high-grade NHL and IHC was positive for CD 20, BCL-2 and MUM-1 with focal expression of BCL-6, negative for MYC with a Ki 67 of 80%, suggestive of diffuse large B cell lymphoma (ABC subtype).
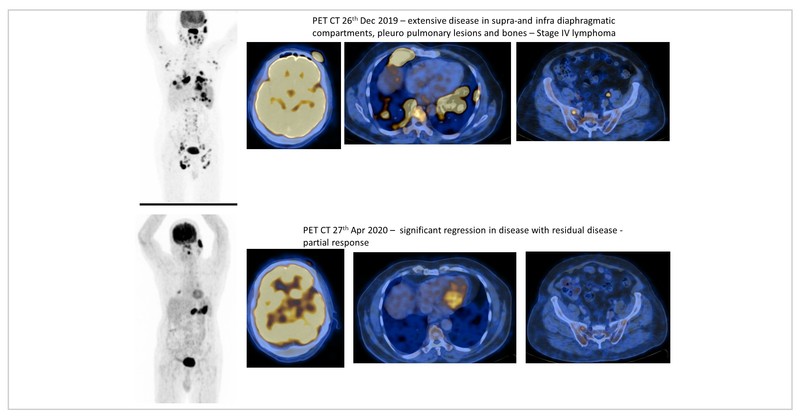
Baseline PET/CT showed hypermetabolic left anterior scalp thickening (SUV max of 25.2), with thickening of nasopharynx (SUV max of 42.8). Also seen were FDG-avid, multiple cervical, axillary, deep pectoral, right hilar, and subcarinal with SUV max of 22.3 in the left axillary region. Multiple pleuropulmonary nodules were also seen. There was splenomegaly (14.4 cm) with multiple focal hypodense lesions (SUV max of 30.3). Multiple enlarged abdominal LN with max size of 3.6 x 2.5 cm with SUV max of 36.5 in the right inguinal region. It was staged as IV E disease. His R-IPI score was 4/5 (high risk).
Diffuse large B-cell lymphoma (DLBCL) is the most common lymphoid malignancy worldwide. Its incidence increases with age and about 40% of cases occur in patients over 70 years. Tolerance to chemotherapy is relatively poor in the elderly as compared to younger patients and hence they are treated with lower doses of standard chemotherapy.
Prior to deciding on which chemotherapy regimen, it is important to do a comprehensive geriatric assessment (CGA) to functionally assess the patient’s ability to tolerate chemotherapy. The Charlson comorbidity score and the Cumulative Illness Rating Score for Geriatrics should be used. This assessment is vital as co-morbidities increase with age and there is a 6 to 12% risk of mortality with standard R-CHOP and increased risk of cardiac toxicity in older individuals. However, following CGA, a select subset of patients may be eligible for standard R-CHOP doses with growth factor support. But, most oncologists in India prefer to use R-mini CHOP with growth factor support as it is well tolerated and gives reasonable results (2-year progression-free and overall survival rates of 47% and 59%, respectively).
The use of prephase steroids for a week with single dose of vincristine with cyclophosphamide is rather useful in reducing the treatment related toxicity by 50%. In case the patients are not eligible for anthracyclines, R-GCVP (Rituximab, Gemcitabine, Cyclophosphamide, Vincristine, and Prednisolone) and R-CVP (Rituximab, Cyclophosphamide, Vincristine, and Prednisone) may be considered. Though R-EPOCH is less cardiotoxic due to infusional Doxorubicin, elderly patients may not tolerate this regimen as it causes more febrile neutropenia.
What is the role of CNS prophylaxis in this patient?
This patient has nasopharyngeal involvement with a CNS IPI score of 3 which will qualify for CNS prophylaxis. A minority of patients with DLBCL will have relapse within the central nervous system (CNS), which has extremely poor outcomes.
Recently, the German High Grade NHL Study Group (DSHNHL) and British Columbia Cancer Agency (BCCA) validated the CNS International Prognostic Index (CNS-IPI) as a tool to predict risk of CNS recurrence. Patients are stratified as having low (0-1 points), intermediate (2-3 points), or high (4-6 points) risk disease, which predicted 2-year rates of CNS relapse of 0.8%, 3.9%, and 12%, respectively.
The CNS relapses are more with double hit lymphomas and certain double expressor lymphomas. Hence prophylactic CNS-directed therapy should be considered for all patients with double- or triple-hit lymphoma, DLBCL patients with a high-risk CNS-IPI score (4-6 risk factors), and intermediate-risk patients (2-3 risk factors) who are ABC-subtype with dual expression of MYC and BCL2. CNS prophylaxis is also recommended in primary testicular DLBCL, parameningeal/para spinal sites and HIV associated DLBCL.
There is a lot of debate of intrathecal vs systemic Methotrexate. The RICOVER-60 , MinT trial and the UK NCRI trials did not demonstrate benefit with intrathecal Methotrexate. The role of systemic Methotrexate is only indirect evidence from the phase III GELA trial comparing CHOP-21 vs. ACVBP regimen.The ACVBP arm included four doses of intrathecal methotrexate, plus two infusions of high-dose systemic Methotrexate at 3000 mg/m2. There were fewer CNS recurrences in the ACVBP arm compared to the CHOP arm (2.7% versus 8%; P=0.004), as well as an overall survival benefit. This may have been contributed by intravenous Methotrexate rather than intrathecal Methotrexate, however this was in the pre-Rituximab era.
The guidelines suggest IV Methotrexate dose of 3500 mg/m2 on day 15 of the 21-day R-CHOP cycle for up to a total of three doses, usually administered in alternating R-CHOP cycles or three or four doses of intravenous Methotrexate immediately following completion of R-CHOP. Trials evaluating IV Methotrexate doses of 1000 to 1500 mg/m2 are being undertaken in elderly patients. Results if favourable may favour consideration of this intermediate dose of methotrexate in elderly patients in the future.
Case continuation
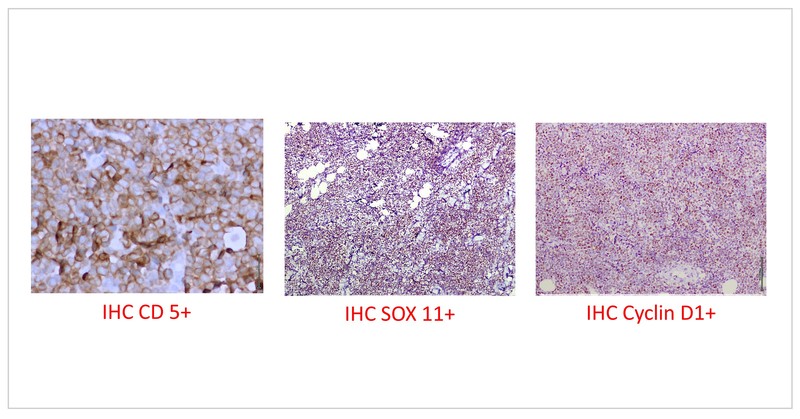
The patient received 2 cycles of R-mini CHOP with limited clinical response with development of additional new swellings on scalp. Hence the HPE block was submitted for additional investigations. The additional markers showed CD 5 positivity and CD 23 negativity with lymphoid cells expressing cyclin D1 and SOX 11. The FISH studies were also positive for 11;14 translocation. The final diagnosis was a blastoid mantle cell lymphoma, high risk MIPI (score of 8.7).
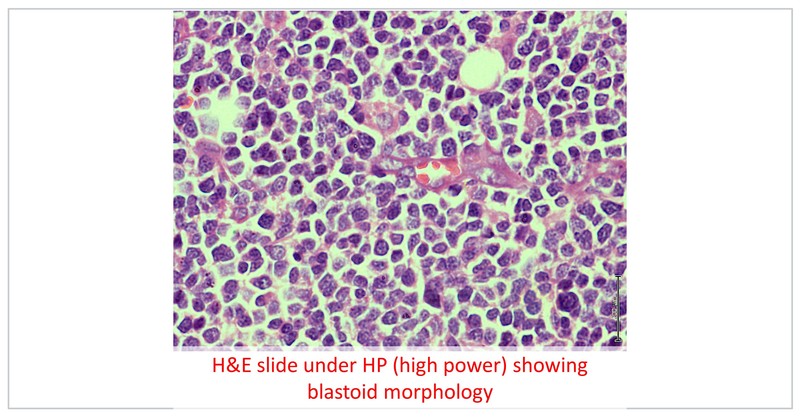
The StIL trial which comprised 15–20% of MCL patients III demonstrated non-inferiority of Bendamustine–Rituximab (BR) to R-CHOP and this regimen is well tolerated even in elderly. However, this patient had not responded to R-mini CHOP and the likelihood of responding to BR in this scenario is rather limited. The role of maintenance Rituximab following BR is rather controversial, however it is established following R-CHOP. Continuing R-mini CHOP may not be an ideal option as the patient has not responded. R-HperCVAD and R-DHAP are regimens for younger and fit patients. VcR-CAP may not be a suitable regimen for elderly patients.
A phase 2 trial of Rituximab with Lenalidomide with a median age of 65 years showed a 2-year progression-free survival was estimated to be 85% and the 2-year overall survival 97%. The response to treatment was associated with improvement in quality of life. This regimen may be considered in this patient. There is also growing evidence to suggest that Ibrutinib can be used a maintenance following BR induction.
The SHINE trial results which is looking at BR+ Ibrutinib in elderly patients will be out next year. This may be a well-tolerated regimen in elderly patients.
A recent phase 2 trial looked at Ibrutinib with Rituximab in combination for elderly MCL. However, blastoid MCL was excluded*. The ORR was 95% and CR of 69%.
In this trial, patients were treated with Ibrutinib 560 mg orally daily for each 28-day cycle, with therapy continued until disease progression, or until therapy was stopped for any other reason. Patients also received intravenous Rituximab 375 mg/m2 on days 1, 8, 15 and 22, plus or minus one day for cycle 1, on day 1 of cycles 3-8, and on day 1 of every other cycle for up to 2 years.
This is still in its early days and may be a good regimen for elderly untreated MCL patients. In view of blastoid MCL and high MIPI, this patient also requires CNS prophylaxis.
This patient was initiated on Ibrutinib with Rituximab and at 2 months interim PET/CT showed complete metabolic response. It is planned to follow the regimen as mentioned above with incorporation of CNS prophylaxis.
PET’s ability to detect disease in bone marrow and GIT is relatively poor resulting in low sensitivity although its positive predictive value is high making it highly specific for marrow and GIT involvement. iPET, also called the interim PET is useful for assessment of tumour response in HL with satisfactory results. However, iPET in DLBCL and MCL has high rates of false positivity causing inaccurate prognostication. Deauville scoring system is commonly used in iPET with a 5-point scoring system from 1 to 5, based on the lesion SUV compared to the mediastinal as well a sliver uptake. Some investigators are evaluating the value of Deauville ratio which is the ration of SUV in the lesion to the SUV in liver, with a value of 1.4 being a robust prognosticating factor.
RECIST criteria is used to assess treatment response in solid tumors using unidimensional measurements. This cannot be used in lymphoma. Lugano criteria is based on [18F]2-fluoro-2-deoxy-D-glucose positron-emission tomography (FDG-PET – Deauville score)) or bidimensional tumor measurements on computed tomography (CT) scans. Response evaluation criteria in lymphoma (RECIL 2017) is an attempt to harmonise with RECIST and uses uni-dimensional measurement of up to 3 target lesions and also accommodates PET findings for categorisation of complete response.
Response categories include CR, PR, MR (minor response), SD and PD; the additional response category of minor response is included (reduction in the sum of longest diameters between 10 to 30%) with progressive disease similar to RECIST 1.1 with more than 20% increase in the sum of diameters, a stable disease defined as decrease <10% to increase ≤20% in the sum of longest diameters, partial response being 30% or more reduction but not CR and progressive disease being 20% or more increase. CR is also assigned when the reduction is 30% or more with normalisation of PET (Deauville 1, 2 and 3).
*Note: Though blastoid mantle cell lymphoma patients were excluded in the phase 2 trial, in this particular case, the patient with blastoid mantle cell lymphoma was initiated on this regimen due to lack of alternative treatment options. The treatment was initiated after a detailed discussion with the patient.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
Dr. Vishwanath Sathyanarayan is a Medical Oncologist from Apollo Hospitals, Bangalore with a fellowship in Lymphoma and Myeloma from MD Anderson Cancer Center.
Dr. Govindarajan MJ is an Interventional Radiologist and the Head of Oncoimaging at Apollo Hospitals, Bengaluru.
The authors would like to acknowledge Dr. Premalatha, Senior Pathologist for the images and details provided.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries