Even as scientists across the world scamper to find a vaccine or cure to the novel coronavirus, another drug which has been tried selectively is showing promising results.
For our comprehensive coverage and latest updates on COVID-19 click here.
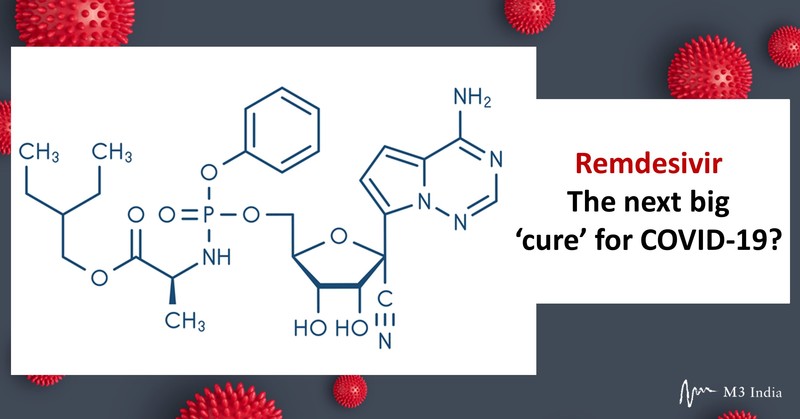
When Remdesivir was first discovered, it was created with the intention of being used as a generic antiviral medication. Now almost over a decade later, scientists have discovered that the drug may possibly be used to target the coronavirus outbreak.
What is this drug and how beneficial is it to treating someone afflicted with coronavirus disease (COVID-19)?
