Stepwise approach to pain management in chronic kidney disease (CKD)
M3 India Newsdesk Feb 12, 2020
Dr. NK Hase details on the principles of pain management, steps in pharmacological treatment of pain in chronic kidney disease, and the use of WHO's analgesic ladder, effective in CKD patients as well.

Patients with advanced chronic kidney disease have a high burden of physical and psychological symptoms. Pain is the most common symptom reported in 60 to 70% of pre-dialysis chronic kidney disease (CKD) patients and 40 to 60% patients receiving dialysis. It can be severe in CKD patients, greatly reducing the quality of life.
The International Association for Study of Pain defined pain as, 'an unpleasant sensory and emotional experience associated with actual or potential tissue damage'. This definition suggests pain is a multidimensional phenomenon with physical and psycho-social components.
Successful management of pain will require both pharmacological as well as non-pharmacological approaches. The non-pharmacological therapies include aerobic exercise, stretching, massage, acupressure, acupuncture, application of heat/ice, behavioural therapies such as cognitive behavioural therapy, biofeedback, relaxation techniques, and counseling guided by imagery and mindfulness-based stress reduction.
The focus of this review is pharmacological management of acute and chronic pain in patients with CKD.
Steps in pharmacological management of pain
Assessment of pain is the key factor to decide pharmacotherapy. It is always recommended to determine the underlying cause and to treat the cause to get permanent relief. History and examination target at:
- Determining the type of pain- nociceptive, neuropathic/neuralgic or combined
- Intensity of pain- acute or chronic pain
- Effect of pain on quality of life (QoL), functional status, social functioning and physiologic effect
Type of pain
Nociceptive: It results from damage to the skin, muscles and other tissues, causing stimulation of sensory receptors. It is often described by the patient as aching, dull throbbing, cramping due to pressure or a colicky pain. Visceral may be poorly localised, somatic is usually well localised.
Neuropathic/Neuralgic: It results from damage to the nervous system in either dysfunction or pathogenic change. It is often described as burning, shooting, stabbing, numbness, and tingling along the nerve. It may be associated with spontaneous pain, hyperalgesia and allodynia. Examples include peripheral neuropathy, severe pain associated with limb ischaemia and calciphylaxis. The pain shows poor response to analgesics and typically requires adjuvant therapy such as gabapentin, carbamazepine and tricyclic antidepressants.
Assessment of pain
Use system SOCRATES to capture all aspects of pain which will help in diagnosis and management.
- Site of pain: Where is the pain? What is the pattern of joint involvement? Is it visceral pain, somatic, or along the nerve?
- Onset: When did the pain start? Is it acute, sub-acute, chronic, constant, intermittent, or episodic?
- Character of pain: Is it burning, shooting, stabbing, colicky, or a dull ache?
- Radiation: Is the pain felt anywhere else?
- Associated features: Is there accompanying nausea, vomiting, altered bowel movement, weight loss, or fever?
- Timing and pattern: Is it worse at any time of the day? Is it associated with any particular activities? Is it acute, chronic, or recurrent?
- Exacerbating and relieving factor: Does the pain increase during movement; does it lessen upon eating or resting?
- Severity: Assess the intensity of pain on a 1 to 10 scale- 0 for no pain at all, and 10 for the worst pain imaginable, 1 to 4 for mild, 5 to 6 for moderate, and 5 to 10 for severe. Record interference with sleep or usual activities and impact on quality of life.
Essential principles for pharmacological management of pain
The WHO analgesic ladder is commonly used to manage pain in cancer all over the world. Recent studies have shown that the WHO analgesic ladder is likely to be effective in CKD patients as well.
The principles are as follows:
- By mouth: Oral administration is safest and the preferred route for patients who can swallow and absorb.
- By the clock: If the pain is constant or continuous, analgesic should be given regularly. Additional 'breakthrough' or 'rescue' medication should be given on an as-needed basis in addition to regular doses.
- By the ladder: Pharmacological management should proceed stepwise from non-opioids to low-dose opioids as per severity and response to pain. The drug should be used in full-tolerated dose before moving to next levels.
- For the individual: Therapy should be individualised according to tolerability and acceptability.
- With attention to detail: Frequent assessment for efficacy and toxicity, dose adjustment according to assessment, and active management of side effects should be performed.
Initial analgesia is selected according to the intensity of pain. When a visual analogue scale of 0-10 is used:
- Mild pain- Score 1-4 and step 1 on the WHO analgesic ladder
- Moderate pain- Score 5-6 and step 2 on WHO analgesic ladder
- Severe pain- Score 7-10 and step 3 on WHO analgesic ladder
At all stages, appropriate adjuvant treatment can be added. If pain is not adequately controlled at step 1, move up to next step of the ladder. Or if you are already on step 3, titrate the strong opioid upwards in relief with monitoring side effects. Start low and go slow.
WHO analgesic ladder Step 1 and 2
Mild pain (score 1-4)
Step 1 recommendation: Non-opioid (paracetamol) ± adjuvant
Paracetamol: It is overall considered as one of the safest drugs to use for the treatment of pain in renal patients including in advanced CKD stage 4-5 without increasing the disease progression rates as long as dosing is below the minimal daily dose. It is metabolised in the liver to five inactive metabolites and does not result in platelet inhibition or gastrointestinal irritation. It is a dialysable compound and half life is prolonged in patients with renal failure.
Recommended dose: 1 g four times a day.
Note: When using products that combine opioids with acetaminophen (paracetamol), do not exceed 3.2 g of dose per day to avoid hepatotoxicity.
Adjuvant: This includes medications such as anti-convulsants, gabapantin, pregabalin for neuropathic pain. It may also include medication to manage an adverse effect of an opioid or enhance analgesia such as steroid for pain from bone metastases.
Non-steroidal anti-inflammatory drugs (NSAIDS): They act by COX inhibition, decreasing prostaglandin synthesis, and decreasing pain and inflammation. The afferent arteriolar vasoconstriction leads to decrease in glomerular filtration rate (GFR), oedema due to sodium and water retention, worsening of hypertension, and hyperkalaemia. They can also cause acute kidney injury- acute tubule-interstitial nephritis and nephrotic syndrome and lead to the progression of CKD. There may be non-renal side-effects too, like increased risk of gastrointestinal bleed and decreased anti-hypertensive effects of several medication. They should be avoided in CKD and maybe considered at end-of-life for relieving the pain.
Note: Ibuprofen is considered a safe option in renal insufficiency or dialysis patients. Also, a topical NSAID can be used safely for relieving acute or chronic pain in CKD.
Opioids for mild to moderate pain + non-opioid ± adjuvants
Step 2 recommendation: Recommended: Paracetamol + Tramadol Avoid: Codeine, Dihydrocodeine
Tramadol: It is a Serotonin Norepinephrine Reuptake Inhibitors (SNRI), which interferes with the pain pathway in dorsal horn and has a dual mechanism of action (opioid + SNRI). It is metabolised in the liver to odesmethyltramadol (ODT), an agonist-like opioid. The enzyme CYP2D6 is involved in metabolism but genetic polymorphism may result in unpredictable metabolism.
The concentration of Tramadol may be 20% higher in 'poor metaboliser' versus individuals who have multiple copies of CYP2D6 gene ultra-rapid metaboliser. Drugs like cinacalcet, paroxetine, fluoxetine, bupropion, strong inhibitor of CYP2D6 interact with Tramadol. Sedation, nausea, vomiting, decreased threshold for seizures, serotonin syndrome and hypoglycaemia have been reported as adverse reactions/side-effects.
Dose reduction is recommended in advanced stages of CKD and it is recommended to avoid sustain-release preparations and instead use immediate-release preparations.
Dosage should be as follows:
- Patient on dialysis- 50 mg TDS three times a day
- Patient not on dialysis- 50 mg twice a day
Codeine: It is a weak opioid, metabolised by the enzyme CYP2D6 in the liver into its active metabolite morphine. It shows variable response in rapid metabolisers, which may result in life-threatening or fatal respiratory depression due to high levels of plasma morphine and poor analgesic response in poor metabolisers. Both codeine and its metabolites are excreted by the kidney and accumulated in patient with kidney failure.
- Recommendation: Avoid in stage 4-5 CKD and in patients on dialysis
Opioids for moderate-severe pain + non opioid ± adjuvant
Step 3 recommendation: Tramadol immediate release 50 mg + Paracetamol upto 3.2 g/day+ Adjuvant as indicated
Opioids
When to initiate?
- When pain relief with other treatments has not been adequate
- When pain is moderate to severe in nature
- When quality of life and function is severely affected by pain
How to initiate?
Start with lower than recommended doses and slowly titrate up the dose while extending the dosing interval. Start low and go slow. This will help to reduce the adverse effects such as respiratory depression and hypotension.
What are the risks?
- Prolonged use of opioids leads to physical dependence and stoppage of medications leads to withdrawal syndrome which is worse than pain
- Watch out for nausea and vomiting
- Patients may need antiemetic before giving opioids
- Constipation is almost universal and patients should be provided with a regular laxative
Note: Patients on dialysis, who take opioids have more falls, altered mental status, fractures and may have higher mortality. Always decide risks and benefits before starting opioids.
What are opioid options?
Morphine and codeine: They should be avoided in CKD. They are metabolised to morphine-3-glucuronide (M3G) and M6G. M6G is very potent and crosses the blood-brain barrier. Metabolites are extracted by kidneys. In CKD, these metabolites accumulate increasing CNS toxicity (sedation, confusion and myoclonic jerks). Morphine is dialysable. When patient is dialysed, M6G can reaccumulate (rebound) and potentially continue to cause CNS toxicity (particularly altered sensorium may be prolonged).
Hydromorphone, fentanyl, methadone and buprenorphine are recommended for use in patients with CKD.
Hydromorphone: It is a synthetic mu receptor agonist. Oral administration works best and it is 5 to 7 times more potent than morphine. It is also tolerated better than morphine. Oral bioavailability is 5 to 35%. It displays extensive first pass hepatic metabolism and is metabolised to H3G which has no analgesic activity but possibly causes neuro-excitation, agitation, confusion, and hallucination.
Hydromorphone does not accumulate in ESRD and is not removed appreciably by dialysis. Dosing recommendations start at 0.5 mg per orally or 0.2 mg subcutaneously. The retrospective review and expert consensus suggestion for dialysis patients is hydromorphone immediate release 1.3 mg 4 to 6 hourly and 1.3 mg as needed if pain breaks through. The total daily dose should be kept low by using transdermal fentanyl when >6 mg/day is needed. Accepted practice is to substitute transdermal fentanyl 25 mcg/h patch when more than nine doses of hydromorphone (1.3 mg) is used in 24 hours and the pain continues. Monitor for hypotension and respiratory depression.
Fentanyl: It is a potent synthetic mu agonist and is 50 to 100 times as potent as morphine. It causes less histamine release, lower incidence of constipation and great haemodynamic stability as compared to morphine. Oral bio-availability is poor and it usually has to be administered intravenously or transdermally. It displays hepatic metabolism with 10 to 20% excreted by the kidneys. Metabolites are inactive and the accumulation is not clinically significant in renal failure. They are also not removed significantly by haemodialysis.
Fentanyl is not recommended in opioid-naïve patients. When converting from hydromorphone daily, 6 to 8 mg oral hydromorhone daily can be converted to 12 mcg/hour transdermally every 72 hours.
Buprenorphine: It is a potent semi-synthetic, partial mu agonist/kappa antagonist. It is sublingually 30 to 60 times as potent as morphine. Oral bio-availability is poor and it is administered effectively, either sub-lingually or transdermally. It is metabolised in the liver to buprenorphine 3-glucuronide and norbuprenorphine (NorB), both of which accumulate in renal failure- 15% biliary excretion.
B3G is inactive and NorB has minor analgesic activity, and has been known to cause respiratory depression in rats. There is a lack of evidence about long-term use in ESKD. However, there are theoretical reasons why it might be a good choice in kidney disease and expert consensus is that it can be used cautiously, particularly, the low strength transdermal preparations.
- Dose recommendation: Start at 5 mcg/h transdermally every 7 days.
Methadone: The synthetic opioid active is a mu opioid receptor, which shows some activity as N-methyl-D-aspartate (NMDA) receptor antagonists and therefore, has a possible role in neuropathic pain. It is excreted mainly in the faeces but also metabolises in the liver to inactive metabolites. It is not removed by dialysis, and there is no accumulation in CKD. Methadone may be safe and effective for use in patients with advanced CKD.
Neuropathic pain management
Diabetic neuropathy, carpel tunnel syndrome, ischaemic neuropathy due to steal syndrome in CKD can present with neuropathic pain.
First-line- Gabanergic drugs
Gabapentin and pregabalin are used as first-line drugs in neuropathic pain. Gabapentin is a structural analogue of gamma aminobutyric acid (GABA). It reduces glutamate-mediated pain signaling. Its specific mechanism of action is to act as alpha 2 delta ligands that binds the L-type voltage-gated calcium blocker in the CNS and reduces transmission of nerve pain signaling by reducing glutamate. They are cleared by kidneys, unchanged. As GFR decreases their elimination is reduced.
CKD patients are at increased risk for neurotoxicity manifested as somnolence, dizziness, myoclonus, drowsiness, and altered sensorium. Doses must be reduced based on eGFR. It is recommended to start 100 mg post dialysis in haemodialysis patients and 100 mg every 2nd night in non-dialysis CKD patients with eGFR <15 ml/min.
Pregabalin is also an anti-epileptic that is used to treat refractory neuropathic pain. Pregabalin in renally cleared unchanged. It needs adjustment according to eGFR. Recommended dose among patients with eGFR is less than 15 ml/min. Pregabalin may be effective as low as 25 mg each, post HD session or 25 mg every 2nd day in CKD patients, not on dialysis. Gabapentin and pregabalin are found to be effective in treating various symptoms in ESKD like pruritus, restless leg syndrome, and poor sleep.
Second-line- Tricyclic antidepressents (TCA)
They are considered as second-line therapy for neuropathic pain in CKD. Patients who are not responding to GABAnergic, gabapentin, pregabalin are usually switched to TCA. Side effects include dry mouth, orthostatic hypotension and somnolence due to anticholinergic histaminergic, and adrenergic activity. Amitriptyline has been the most commonly-used TCA for chronic neuropathic pain. Other drugs which have been used successfully include duloxetine, doxepin, imipramine, nortriptyline and desipramine. Amitriptyline can be started at 10 mg orally, daily and gradually increased to maximum tolerated dosage. The onset of analgesia may be after a week.
Management of other pain syndromes
- Colic-bowel obstruction: Hysocine butyl bromide should be given parenterally
- Renal colic: NSAIDs should be the first choice unless absolute contraindication. Hyoscine butylbromide is the second choice. The patient may need opioid; first choice is fentanyl.
- Muscle spasm: Benzodiazepines, Diazepam if the patient is able to swallow. Clonazepam single dose should be given at night. Avoid Baclofen in CKD as it can produce profound drowsiness and coma.
Doses adjustment of pain medications used in CKD
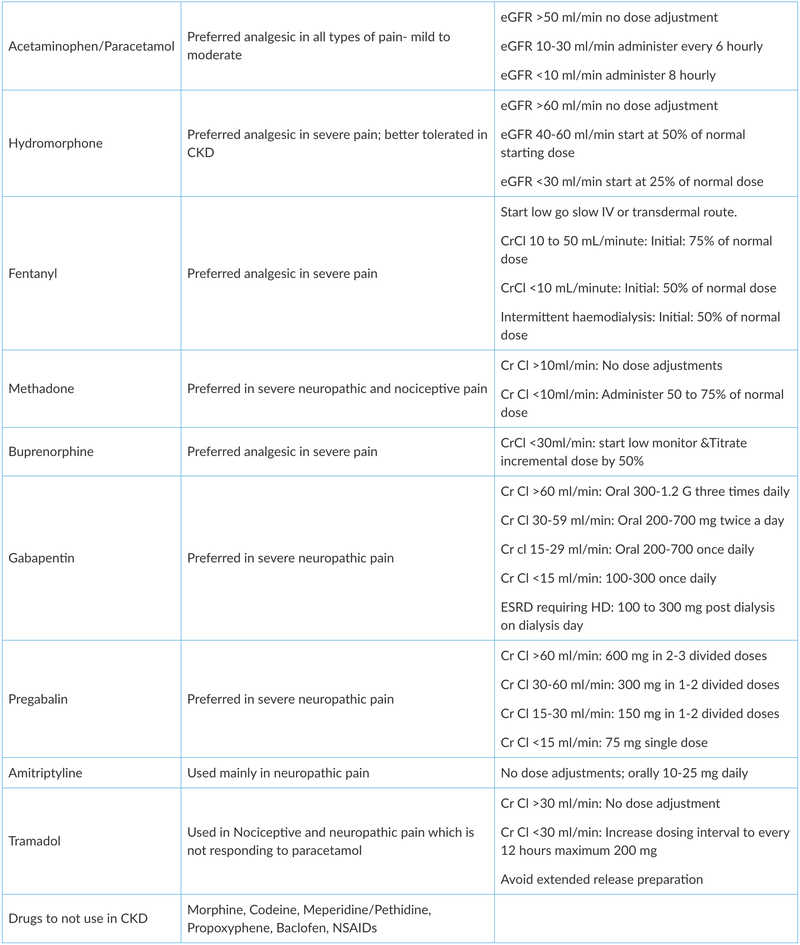
Pain is common and a distressing symptom reported by patients with CKD. It affects the quality of life. Pain management in CKD is challenging as commonly used drugs require careful handling. Their pharmacokinetics are altered in patients with CKD. Therefore, a stepwise approach is required to achieve the goals of pain relief with maximum benefit and least risk.
Click here to see references
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The writer, Dr. Niwrutti Hase is a Director Clinical Nephrology & Kidney transplant working in Jupiter Hospital, Thane.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries