Virtual Tumour Board: Multidisciplinary panel discusses case of 42-year old patient with early breast cancer
M3 India Newsdesk Aug 27, 2019
Listen to the audio file at your convenience and read the text of a panel discussion involving a multidisciplinary team- surgical, imaging, radiation, and medical experts in Oncology deliberating on the case of a 42-year old patient diagnosed with early breast cancer (EBC). The panelists have put forth their respective opinions on the treatment approach based on the latest trials and guidelines in EBC.
Introduction: Dr. Vishwanath
As we are aware, cancer treatment involves a multi-disciplinary approach involving medical, radiation, surgical oncologists, radiologists and pathologists. Here we discuss early breast cancer (EBC); a case of Her2 positive EBC. We hope this educational series will be a valuable addition to practising oncologists and physicians as it has also incorporated an Indian perspective.
I would like to introduce my panel which includes myself, Dr. Vishwanath S, consultant medical oncologist at Apollo Hospitals, Bangalore. The other members include Dr. Anil Kamath: Surgical Oncologist, Apollo Hospitals, Bangalore, Dr. Govindarajan: Head, Onco-imaging, Apollo Hospitals, Bangalore and Dr. Bindu Venugopal: Radiation Oncologist, Kidwai Institute of Oncology, Bangalore.
Case presentation: Dr. Anil Kamath
- 42-year old premenopausal lady
- Lump in the breast of 4 months duration
- No family history of cancer
- O/E: Approximately 2 cm lump in the right breast upper and outer quadrant
- No axillary lymph nodes
- No evidence of distant metastases
Work up: Dr. Anil Kamath and Dr. Govindarajan
Mammography understanding: Dr. Govindarajan
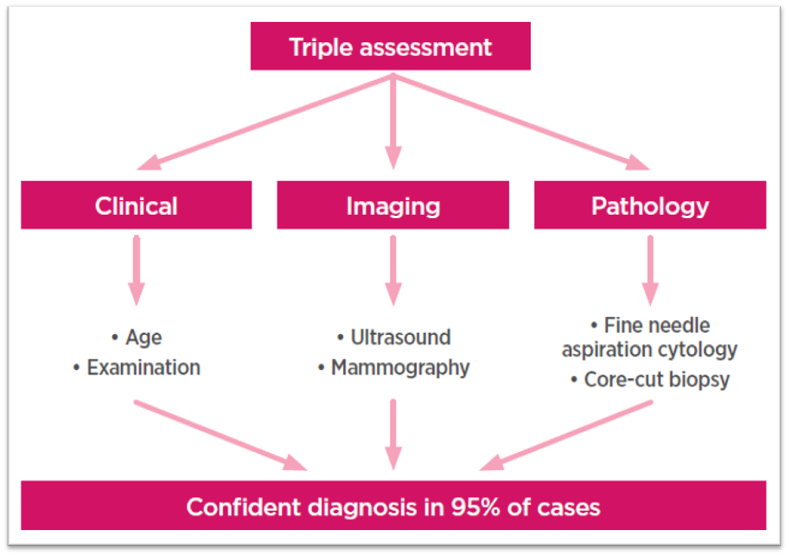
- Digital mammogram demonstrates a spiculated density with architectural distortion in the upper outer quadrant of the right breast (straight long arrow)
- A high resolution ultrasound scan demonstrates a spiculated hypoechoic mass (curved arrow) with distal acoustic shadowing (short straight arrow)
How to proceed?: Dr. Anil Kamath
- FNAC: Can give diagnosis but will not provide tissue for immunohistoshemistry
- Trucut biopsy: More suitable as it provides tissue to do immunohistochemistry
Procedure of core biopsy: Dr. Anil Kamath and Dr. Govindarajan
- Done under local anesthesia
- Outpatient procedure
- Preferred method is an image guided core biopsy using a 16G or 18 G gun
- Sufficient sample to be obtained for histopathology, and assessment of receptor status
- High resolution ultrasound of axillary lymph node to look for suspicious metastatic node and if positive (any one or more - cortex thickening, focal or diffuse-more than 3 mm, excessively hypoechoic cortex, hilar effacement, non-hilar cortical vascularity, compression of hyperechoic medulla, size more than 10 mm in short axis and height to length ratio of more than 0.5), core biopsy under ultrasound guidance
- Imaging tool for guidance is generally and most commonly an ultrasound when the lesion is well seen on ultrasound; however a stereotactic biopsy using mammography or an MRI guided biopsy may be performed if the lesions are seen only on these modalities (screening MRI is performed on select high risk women).
Biopsy Result: Infiltrative ductal carcinoma (IDC), grade II ER8+/PR7+/Her2 3+; Ki 67 20-25%
Staging imaging modalities: Dr. Govindarajan
- Incidence of distant metastases is extremely low in early breast cancer.
- F18 FDG PET CT scan is generally not indicated in these patients.
- Bilateral mammography is indicated to rule out multicentricity and cancer in the contralateral breast.
- Usually a chest radiograph and ultrasound abdomen are used.
- However, a minority of patients with T1 breast lesion with no axillary node involvement have presented with distant metastases and hence the recent development of increasing use of PET CT by oncologists as well as patients though not part of any guidelines.
- MRI breast is possibly a significant investigation if the patient is planned for breast conservation surgery.
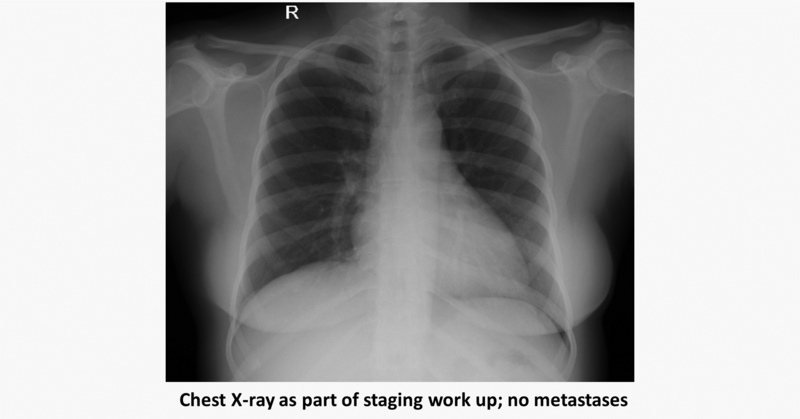
Role of neoadjuvant chemotherapy (NACT) in Her2 3+ EBC: Dr. Vishwanath
Surgical aspects: Dr. Anil Kamath
- Surgery for the primary- Breast conservation surgery/mastectomy
- Surgery for the axilla- Sentinel node biopsy/axillary dissection
- Options for reconstruction
Pathology: Dr. Vishwanath and Dr. Bindu Venugopal
- Invasive ductal carcinoma grade II NOS
- 9x7 mm size tumour
- All margins free of tumour
- Extensive intraductal component present
- 0/8 lymph nodes positive (SLNB)
- ER 7+/ PR 8+/ Her 2 neu 3+
- Ki 67: 20 to 25%
Updated APT trial results: Tolaney et al.
The Adjuvant Paclitaxel and Trastuzumab trial was designed to address treatment of patients with small human epidermal growth factor receptor 2 (HER2)–positive early breast cancer. Phase II study: 410 Patients with HER2-positive breast cancer with tumors 3 cm or smaller and negative nodes received paclitaxel (80 mg/m2) with trastuzumab for 12 weeks, followed by trastuzumab for 9 months.
Findings:
- After a median follow-up of 6.5 years, the 7-year DFS was 93% (95% CI, 90.4 to 96.2), 7-year OS was 95% (95% CI, 92.4 to 97.7).
- With longer follow-up, adjuvant paclitaxel and trastuzumab is associated with excellent long-term outcomes.
PERSEPHONE Trial
The trial aimed to investigate whether 6-month adjuvant trastuzumab treatment is non-inferior to the standard 12-month treatment regarding disease-free survival. In the randomised phase 3 non-inferiority trial, over 4000 patients with HER2-positive early breast cancer, aged 18 years or older, and with a clear indication for chemotherapy, by a computerised minimisation process (1:1), received either 6-month or 12-month trastuzumab delivered every 3 weeks intravenously (loading dose of 8 mg/kg followed by maintenance doses of 6 mg/kg) or subcutaneously (600 mg), given in combination with chemotherapy (concurrently or sequentially).
The primary endpoint was disease-free survival, analysed by intention to treat, with a non-inferiority margin of 3% for 4-year disease-free survival. Safety was analysed in all patients who received trastuzumab. Median follow-up was 5·4 years (IQR 3·6–6·7) for both treatment groups.
Findings:
- 4-year disease-free survival was 89·4% (95% CI 87·9–90·7) in the 6-month group and 89·8% (88·3–91·1) in the 12-month group (hazard ratio 1·07 [90% CI 0·93–1·24], non-inferiority p=0·011), showing non-inferiority of the 6-month treatment.
- 6-month trastuzumab treatment resulted in fewer patients reporting severe adverse events.
- Patients with ER+, use of anthracyclines and sequential chemo did better with 6 months of trastuzumab.
Radiation therapy: Dr. Bindu Venugopal
- EBCTCG group meta-analysis showed that adjuvant RT post BCS reduced 15-year risk of breast cancer death from 25.2% to 21.4%.
- In patients with N0 status the risk reduced from 31% to 15.6%
- Absolute mortality reduction was 3.3%
- One breast cancer death avoided for every four recurrences avoided.
ASTRO guidelines for Whole Breast Irradiation (WBI)
Recommended dose 45Gy/15fr or 46.5Gy/16fr
- The decision for hypofractionation is independent of age, tumour grade, laterality, receptor status and chemotherapy
- Tumour bed boost is given in patients aged <50 years, 50-70 with high grade histology and positive margins
- 10Gy/5fr is recommended as tumour bed boost sequentially irrespective of WBI dose fractionation, dose and histology. 16Gy/8fr for positive margins
- Preferred modality of treatment is 3DCRT with ‘field in field’ to achieve homogeneity
ASTRO guidelines for Accelerated Partial Breast Irradiation (APBI)
These recommendations were largely based on GEC-ESTRO and IMPORT low studies. ‘Suitable group’- females aged >50 years, unifocal disease, <3 cm tumour, excision margins >2 mm, grade 1-2 disease, ER positive, Her 2 negative, N0 status. Preferred modality of delivery- external beam radiation or interstitial brachytherapy in keeping with GEC-ESTRO guidelines.
Surveillance: Dr. Bindu Venugopal and Dr. Govindarajan
- Clinical surveillance- Every three months. Local and regional examination to look for recurrence, monitor lymphedema and rehabilitation advice. Suggest appropriate investigations in lieu with symptoms/signs.
- Imaging– Mammography every six months and followed annually; ultrasound is generally used along with mammography. MRI usage is only on case to case basis and as a problem-solving tool and not routinely performed.
In the coming weeks, look out for articles on breast cancer from different sub-specialty experts (medical, radiation, surgical oncologists and radiologist) highlighting the recent trends and management issues pertaining to their domain.
Disclaimer
This manuscript is an unedited version, published as a service to our readers and other audience with bandwidth limitations that could possibly restrict them from listening to the audio. While it is believed to be accurate, it is not warranted to be so. Divergence in format is to be expected.
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of M3 India. We do not endorse any of the treatment approaches recommended in the panel discussion. Readers are requested to use their own discretion.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries