Checkpoint inhibitors in cancer immunotherapy: Dr. Suresh Advani
M3 India Newsdesk May 01, 2019
Summary
Dr. Suresh Advani elaborates on checkpoint inhibitors, one of the major breakthroughs in cancer research that have delivered exceptionally well in terms of prolonging progression-free survival in patients when other therapy fails.

Up until now cancer treatment revolved around surgery, radiotherapy and chemotherapy and since past 20 years, we have also developed targeted therapy making it 4th type of treatment modality. In the last 10 years, the basic understanding of immunology has allowed us to develop new immune-oncologic therapies employing modification of the patient’s immune system to selectively attack cancer cells, making it the 5th successful therapy.
What are checkpoint molecules?
An important part of the immune system is its ability to tell between normal cells in the body and those it sees as “foreign.” This lets the immune system attack the foreign cells while leaving the normal cells alone. To do this, it uses “checkpoints” – molecules on certain immune cells that need to be activated (or inactivated) to start an immune response.
Immune checkpoints are regulators of the immune system. Both stimulatory and inhibitory checkpoint molecules are present. Cancer cells sometimes find ways to use these checkpoints to avoid being attacked by the immune system. Modulating the checkpoint proteins expressed on the T cell surface is a strategy to enhance the immune system. Checkpoint signalling weakens T-cell activation to avoid autoimmunity and the destructive effects of exaggerated inflammatory response.
Regulation of immune response
The immune system consisting of cellular and humoral responses recognises and eliminates invading pathogens and neoplastic cells. T cells play a vital role in cell-mediated immunity against cancers. The cancer cell expresses a new antigen on the surface of the cell which is recognised as a foreign antigen. Anti-cancer immune response is a self-propagating cyclic process which is initiated with the release of antigens from a cancer cell. These antigens are captured by antigen presenting cells (APC) such as dendritic cells for processing (Fig.1). Dendritic cells present the processed antigens to T cells by expressing them on their cell surface as major histocompatibility complex (MHC) molecules.
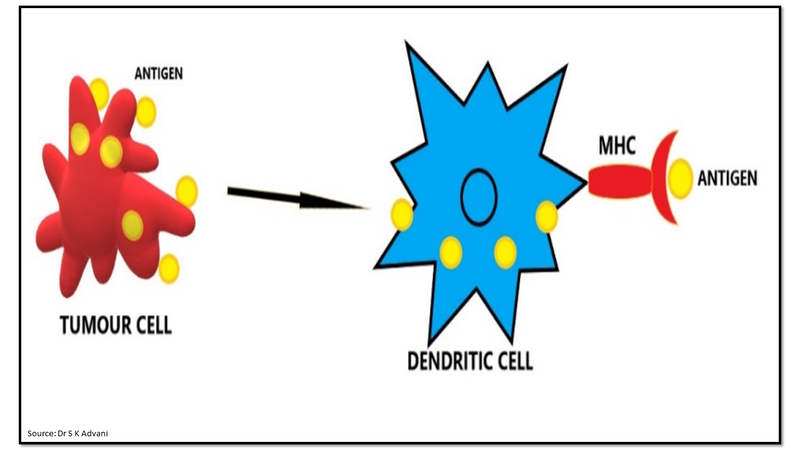
Figure 1: Processing of tumour antigen
The immune response has two phases: the priming phase and the effector phase. During the priming phase, the naive T cells gets activated against tumour specific antigens by the interaction between T cell receptor (TCR) and antigen-MHC. The activation of T cells requires two signals. Signal 1 is antigen specific signal to TCR by specific antigens on MHC molecules that are expressed on APC (Fig.2).
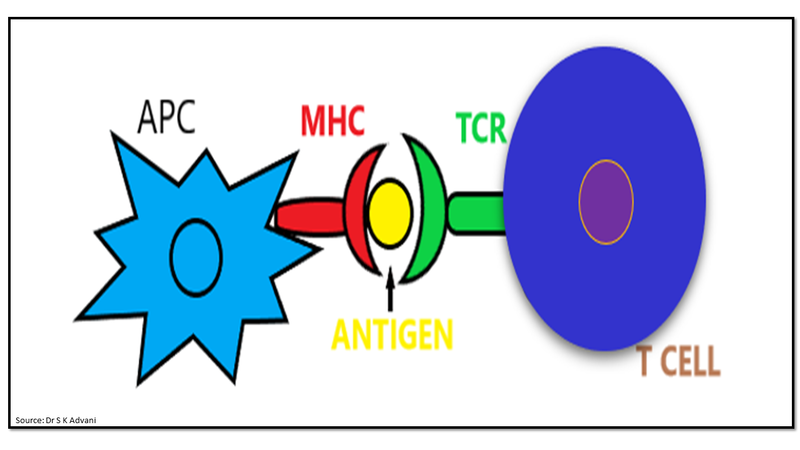
Figure 2: Signal 1- antigen presentation to T cell
Signal 2 is a co-stimulatory signal provided by co-stimulatory molecules like B7 on APCs interacting with CD-28 on T cells to assist in T cell activation (Fig.3).
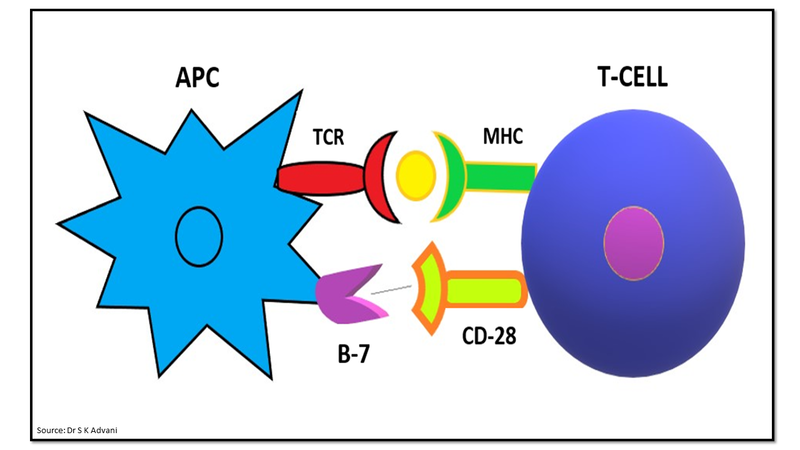
Figure 3: Signal 2- co-stimulatory signalling
The expression of coinhibitory molecule like cytotoxic T lymphocyte antigen-4 (CTLA-4) with strong affinity for B7 predominantly inhibits T cell activation by competing with the CD28+ costimulatory molecule (Fig.4). CTLA-4 is expressed approximately 48 hours after T cell activation and provides dominant negative signaling. The balance between co-stimulatory and co-inhibitory signals determines T cell status.
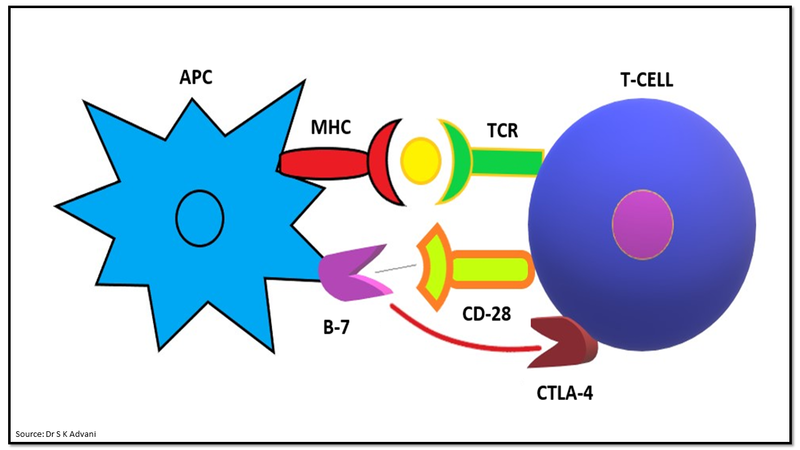
Figure 4: Co-inhibitory signalling by CLTA-4
During the effector phase, T-cell receptor interacts with tumour antigen on cancer cells leading to tumour destruction. This interaction leads to the expression of programmed death-1 (PD-1) molecule on activated T cell. Further interaction between PD-1 on T cells with programmed death ligand 1 and 2 (PD-L1/2) on tumour cells works as an inhibitory signal and produce T cell attenuation (Fig.5).
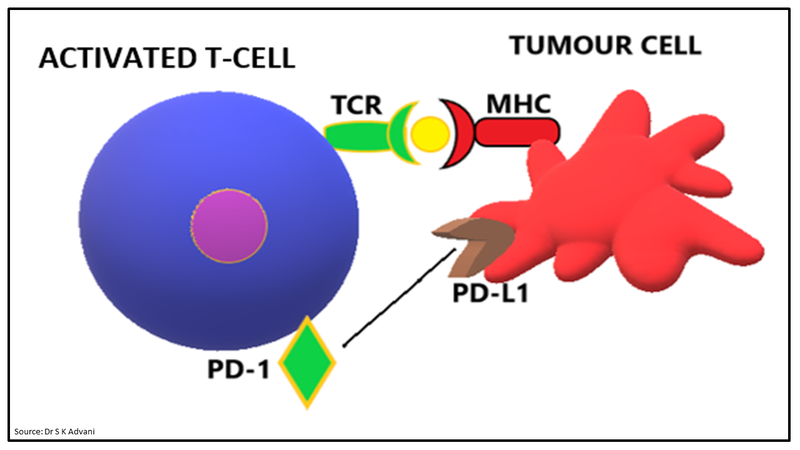
Figure 5: Effector phase- interaction of PD-1 and PD-L1
What are checkpoint inhibitors?
Checkpoint inhibitors are a versatile class of immunomodulatory agents that inhibit/block the inhibitory checkpoint molecules and have been demonstrated to provide clinical benefits in the treatment of cancer. Immune checkpoint blockade removes inhibitory signals of T cell activation, which enables tumour-reactive T cells to overcome regulatory mechanisms and mount an effective anti-tumour response.
There are mainly two immune checkpoint pathways: one for the priming phase - CTLA-4 pathway and one for the effector phase - PD-1/PD-L1 pathway.
- CTLA-4 is a transmembrane glycoprotein that is a homolog of the immune co-stimulatory protein CD28 but with inhibitory effect. Following binding of TCR with antigen, CD28 provides the second co-stimulatory signal for T cell activation by binding to CD80 (B7-1) and CD86 (B7-2) proteins on antigen presenting cells (APCs). CTLA-4 expressed on activated T cells binds B7 proteins with approximately 20 times greater affinity compared to CD28 leading to T cell inactivation.
- PD-1 is a checkpoint protein on T cells. It normally acts as a type of “off switch” that helps keep the T cells from attacking other cells in the body. The interaction between PD-1 on T cells with PD-L1 on some normal and cancer cells leading to T cell attenuation during effector phase.
Immune checkpoint inhibitors are monoclonal antibodies blocking the CTLA-4 or PD-1 mediated inhibitory signals and thus, revitalizing T cell mediated anti-tumour activity (Fig.6). Inhibition of the inhibitory pathway by monoclonal antibody leads to continuous T cell activation against tumour cells and boost the immune response.
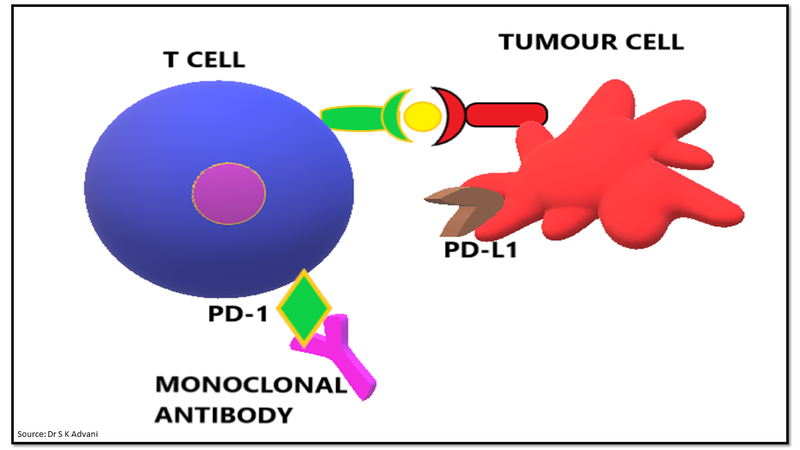
Figure 6: Immune checkpoint blockade by monoclonal antibody
CTLA-4 inhibitors: Ipilimumab (Yervoy)
Ipilimumab is the first discovered anti-CTLA-4 antibody. It has shown effectiveness in metastatic melanoma. The dosage is 3 mg/kg, three-weekly, four doses.1 Ipilimumab has been shown to be superior to chemotherapy and is the first therapy to show a statistically significant and clinically meaningful survival benefit in metastatic melanoma.
Two pivotal Phase III studies have led to ipilimumab being registered for the treatment of melanoma in many countries.2,3 The collective observations with ipilimumab monotherapy are that response rates across the entire patient population are relatively modest; however, about one-fifth to one quarter of the patients have durable responses, extending many months and years.
A recently published pooled analysis of 1861 patients, with 254 patients followed for at least 3 years, showed that the survival curve of patients treated with ipilimumab plateaued at 3 years and practically held steady for up to 10 years follow-up.4 Ipilimumab also showed improvement in patients with non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) when delivered in combination with chemotherapy.
PD-1 inhibitors: Pembrolizumab (Keytruda), Nivolumab (Opdivo), Cemiplimab (Libtayo)
The PD-1 inhibitors nivolumab and pembrolizumab have demonstrated an even higher response rate in initial clinical trials in advanced melanoma patients compared to Ipilimumab.
Nivolumab was approved for first-line treatment for patients with unresectable or metastatic melanoma by the FDA in January 2016. Nivolumab has also been approved for the treatment of advanced NSCLC, metastatic RCC, head and neck squamous cell carcinoma (HNSCC), Hodgkin lymphoma, and urothelial cancer as well as in combination with ipilimumab for the treatment of advanced melanoma. Phase I trials showed that a single dose of nivolumab yielded tumour shrinkage in 28% of melanoma patients, 18% of NSCLC patients and 27% of kidney cancer patients.
PD-L1 inhibitors: Atezolizumab (Tecentriq), Avelumab (Bavencio), Durvalumab (Imfinzi)
- Atezolizumab, the first commercially available PD-L1 inhibitor, is effective in treatment for locally advanced or metastatic urothelial carcinoma after failure of platinum-containing chemotherapy. It is also approved for treatment of NSCLC.
- Avelumab is used for the treatment of adults and pediatric patients 12 years and older with metastatic Merkel cell carcinoma.
- Durvalumab is approved for locally advanced or metastatic urothelial carcinoma after failure of platinum-containing chemotherapy.
Overall, checkpoint inhibitors have revolutionised cancer immunotherapy by demonstrating activity against many challenging cancers. This has led to a paradigm shift in the field of cancer treatment.
Disclaimer- The views and opinions expressed in this article are those of the author's and do not necessarily reflect the official policy or position of M3 India.
The writer, Dr. Suresh Advani is a Senior Oncologist from Mumbai with over three decades of experience in the field.
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries