LOC blood test could detect genetic abnormalities in early pregnancy
University of South Australia News Jun 16, 2018
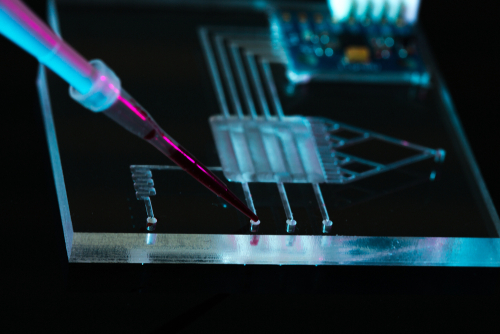
A wide range of fetal genetic abnormalities could soon be detected in early pregnancy thanks to a world-first study led by University of South Australia researchers using lab-on-a-chip (LOC), noninvasive technology.
Biomedical engineers, Dr. Marnie Winter and Professor Benjamin Thierry from UniSA’s Future Industries Institute (FII) and the ARC Centre of Excellence in Convergent Bio-Nano Science and Technology (CBNS), are part of a team of researchers who have isolated fetal cells from maternal blood using a tiny microfluidic device, allowing for improved genetic testing.
The technology breakthrough is published today in Advanced Materials Technologies.
LOC technology integrates laboratory functions on a chip, ranging from a few millimeters to a few square centimeters. The special design of the device allows large volumes of blood to be screened, paving the way for an efficient, cheap, and quick method of separating fetal cells from maternal blood cells.
“We are hopeful that this device could result in a new, noninvasive prenatal diagnostic test able to detect a wide range of genetic abnormalities in early pregnancy from a simple blood sample,” Dr. Winter says.
Currently, prenatal diagnostic tests involve an amniocentesis procedure or taking a sample of cells from the placenta (chorionic villus sampling), both of which carry a risk of inducing miscarriage.
“From about 5 weeks into the pregnancy, fetal cells originating from the placenta can be found in a mother’s bloodstream. Using modern microfluidic technology, we can now isolate these extremely rare cells (about one in a million) from the mother’s white blood cells and collect them for genetic analysis,” she says.
The UniSA researchers, working in collaboration with Dr. Majid Warkiani from the University of Technology Sydney and specialists from the Women’s and Children’s Hospital, SA Pathology and Repromed, adapted the device from one initially developed to isolate tumor cells from the blood of cancer patients.
“Many pregnant women would be aware of the new tests based on circulating fetal DNA that—with a simple blood test—help determine the risk of having a baby with Down syndrome.”
“These tests have revolutionized prenatal care, but they can only detect a small subset of genetic conditions and are not always accurate. We hope this LOC technology will be able to reliably detect a greater range of genetic abnormalities, providing more information to families and health-care providers,” Dr. Winter says.
Professor Thierry, who leads UniSA’s Bioengineering group, says there is significant scope to further develop the LOC concept.
“We are collaborating with industry partners to translate this technology in routine clinical prenatal diagnostics and make it available in the future to screen low- and medium-risk pregnancies,” he says.
Professor Emily Hilder, Director of UniSA’s FII, says: “This research breakthrough is testament to the cutting-edge technology being developed at the FII. UniSA is a leading player in LOC technology thanks to our ANFF-SA micro and nanofabrication facility at Mawson Lakes.”
-
Exclusive Write-ups & Webinars by KOLs
-
Daily Quiz by specialty
-
Paid Market Research Surveys
-
Case discussions, News & Journals' summaries