A new at-home coronavirus test kit just received emergency approval from the US Food and Drug Administration.
For our comprehensive coverage and latest updates on COVID-19 click here.
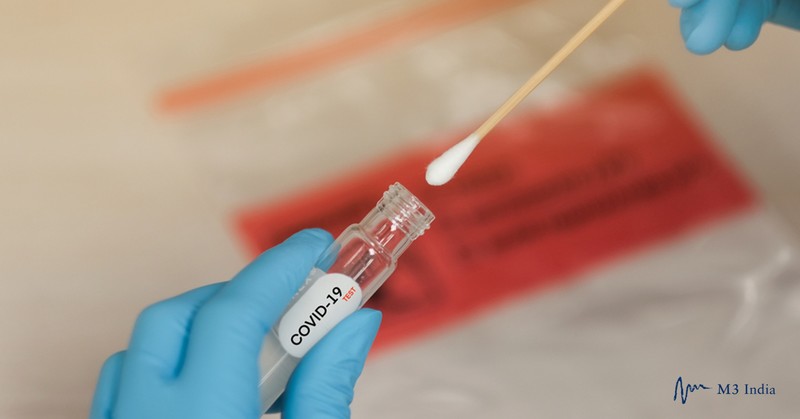
The sample collection kit, from home testing company Everlywell, will be available by prescription only. An online questionnaire reviewed by a healthcare provider will screen potential patients.
The kit includes nasal swabs for taking a sample along with a saline solution-filled tube to store the swab and ship the sample to one of two private companies: Fulgent Therapeutics or Assurance Scientific Laboratories.
Those labs have their own emergency authorization to process Everlywell's coronavirus tests.
"From the moment that you hit the order button, to the moment that you get the test results on your phone or device, that process is designed to take three to five days," Everlywell spokesperson Christina Song told the New York Times.
Song said that the test kits will be available in late May for $135 each.
The FDA has given emergency authorization to two other at-home COVID-19 testing kits. The first was LabCorp's nasal swab kit, which the company recently made widely available after prioritising frontline healthcare workers during its rollout.
The second was a saliva sample kit from Rutgers Clinical Genomics Laboratory.
To show that samples could survive shipping, Everlywell used data from studies by The Bill and Melinda Gates Foundation and UnitedHealth Group. In a statement, the FDA noted that other companies could use the same data in their emergency use requests. That could speed up the process for future test kit approvals.
"The authorization of a COVID-19 at-home collection kit that can be used with multiple tests at multiple labs not only provides increased patient access to tests, but also protects others from potential exposure," Jeffrey Shuren, director of the FDA's Centre for Devices and Radiological Health, said in the statement.
"Today's action is also another great example of public-private partnerships in which data from a privately funded study was used by industry to support an EUA request, saving precious time as we continue our fight against this pandemic."