There is only one approved, specific treatment for COVID-19, the illness caused by the novel coronavirus SARS-CoV-2, albeit with modest efficacy. Numerous experimental or repurposed drugs are under investigation, including the arthritis drug tocilizumab. And one treatment that is more than a century old.
For our comprehensive coverage and latest updates on COVID-19 click here.
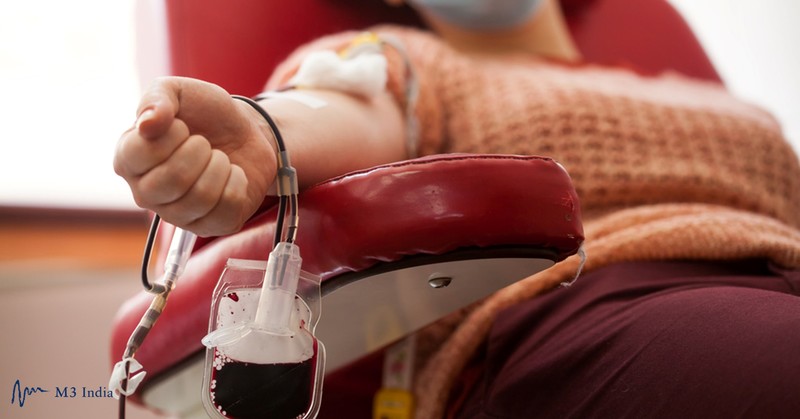
Researchers at University of California San Diego School of Medicine and UC San Diego Health have launched a clinical trial to assess the safety and efficacy of convalescent plasma (CP) to prevent COVID-19 after a known exposure to the virus. CP therapy involves infusing patients with antibodies extracted from the blood of donors who have successfully recovered from COVID-19, with the hope that the resulting boost to their immune systems will shorten the length and reduce the severity of the disease.
The UC San Diego trial is part of a larger, national effort approved by the U.S. Food and Drug Administration. The goal is to create a network of hospitals and blood banks collecting, isolating, processing and testing whether plasma from COVID-19 survivors has therapeutic, preventive value. The national trial is being coordinated by Johns Hopkins University and sponsored by the National Insitute of Health through the Department of Defense.
